Immunome profiling in prostate cancer: a guide for clinicians
- PMID: 39635522
- PMCID: PMC11614818
- DOI: 10.3389/fimmu.2024.1398109
Immunome profiling in prostate cancer: a guide for clinicians
Abstract
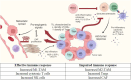
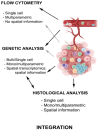
Tumor immune microenvironment (TIME) plays a key role to understand how tumors respond to prostate cancer (PC) therapies and potential mechanisms of resistance. Previous research has suggested that specific genomic aberrations, such as microsatellite instability (MSI) or CDK12 bi-allelic loss can allow PC patients more likely to respond to immune checkpoint inhibitors (ICI) or other immune therapies. However, responses to these treatments remain highly variable even in selected patients. Thus, it is essential to obtain more information about tumor immune cells that infiltrate these tumors, and on their plasticity and interactions, in order to better understand the underlying biology to allow development of new therapeutic strategies. This review analyzes: 1) How interactions among immune cell populations and other cells infiltrating the tumor stroma can modulate the progression of PC, 2) How the standard therapies to treat PC (such as androgen deprivation therapy, new androgen-directed hormone therapy or chemotherapy) may influence the dynamic changes of the immunome and 3) What are the limitations in characterizing the immune landscape of the host´s response to tumors.
Keywords: biomarkers; immunome; immunophenotype; immunotherapy; prostate cancer.
Copyright © 2024 San-Jose Manso, Alfranca, Moreno-Pérez, Ruiz-Vico, Velasco, Toquero, Pacheco, Zapatero, Aldave, Celada, Albers, Fenor de la Maza, García, Castro, Olmos, Colomer and Romero-Laorden.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. (2017) 35:40–7. doi: 10.1200/JCO.2016.69.1584 - DOI - PubMed
-
- Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol Off J Am Soc Clin Oncol. (2020) 38:395–405. doi: 10.1200/JCO.19.01638 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

