The efficacy and safety of remimazolam in painless colonoscopy: a prospective, randomized clinical trial
- PMID: 39635591
- PMCID: PMC11614597
- DOI: 10.3389/fmed.2024.1434767
The efficacy and safety of remimazolam in painless colonoscopy: a prospective, randomized clinical trial
Abstract
Purpose: Remimazolam is a new type of ultra-short-effect intravenous anesthetic, that may provide adequate sedation for endoscopy while causing less cardiovascular or respiratory disturbance than propofol. The aim of this clinical study was to compare the efficacy and safety of two different doses of remimazolam with propofol for sedation during colonoscopy.
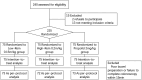
Patients and methods: 225 subjects, aged 18 to 80 years, with American Society of Anesthesiology physical status I-III, were scheduled to undergo colonoscopy. All the subjects were randomly assigned to three groups, Low-Rem group (low dose remimazolam, 0.15 mg/kg, iv, n = 75), High-Rem group (high dose remimazolam, 0.2 mg/kg, iv, n = 75), and Propofol group (propofol 2 mg/kg, iv, n = 75). Every individual in this trial was given nalbuphine hydrochloride (0.2 mg/kg, iv) before administration of remimazolam or propofol. The primary outcome was the success rate of sedation. Haemodynamic parameters and adverse events were recorded to evaluate safety. Satisfaction of sedation from patients, anesthesiologists and gastroenterologists were also recorded.
Results: The success rate of colonoscopy procedure was 100% in both High-Rem and Propofol groups, but it was 89% in Low-Rem group (p < 0.05). Furthermore, the induction time of anesthesia was shorter in Propofol group, when compared to the Low-Rem group and the High-Rem group (p < 0.05). The recovery time in Low-Rem group, High-Rem group, and Propofol group was 2.33, 2.43, and 3.21 min (p < 0.05) respectively, and the time of discharge was 25.00, 25.01, and 27.56 min (p < 0.05) respectively. Simultaneously, the incidence of adverse events such as hypotension, bradycardia, and respiratory depression in the remimazolam groups were significantly lower than that in the propofol group. No significant differences were observed among the three groups in Ramsay scale, BPS-NI scale, and Limb movement classification. Moreover, patients, anesthesiologists, and gastroenterologists were all satisfied with the sedation process.
Conclusion: Remimazolam can be used safely and effectively for colonoscopy. 0.2 mg/kg remimazolam and propofol have the same sedation success rate and more stable hemodynamics and fewer side effects than propofol.
Clinical trial registration: ChiCTR2100054053.
Keywords: anesthesia; colonoscopy; propofol; remimazolam; sedation.
Copyright © 2024 Shi, Zhang, Hu, Hou, Hu, Dai, Zhou, Qi, Li, Wang, Wang, Liao and Qian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Borkett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, et al. . A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. (2015) 120:771–80. doi: 10.1213/ANE.0000000000000548, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

