Transcriptional delineation of polysaccharide utilization loci in the human gut commensal Segatella copri DSM18205 and co-culture with exemplar Bacteroides species on dietary plant glycans
- PMID: 39636128
- PMCID: PMC11784079
- DOI: 10.1128/aem.01759-24
Transcriptional delineation of polysaccharide utilization loci in the human gut commensal Segatella copri DSM18205 and co-culture with exemplar Bacteroides species on dietary plant glycans
Abstract
There is growing interest in members of the genus Segatella (family Prevotellaceae) as members of a well-balanced human gut microbiota (HGM). Segatella are particularly associated with the consumption of a diet rich in plant polysaccharides comprising dietary fiber. However, understanding of the molecular basis of complex carbohydrate utilization in Segatella species is currently incomplete. Here, we used RNA sequencing (RNA-seq) of the type strain Segatella copri DSM 18205 (previously Prevotella copri CB7) to define precisely individual polysaccharide utilization loci (PULs) and associated carbohydrate-active enzymes (CAZymes) that are implicated in the catabolism of common fruit, vegetable, and grain polysaccharides (viz. mixed-linkage β-glucans, xyloglucans, xylans, pectins, and inulin). Although many commonalities were observed, several of these systems exhibited significant compositional and organizational differences vis-à-vis homologs in the better-studied Bacteroides (sister family Bacteroidaceae), which predominate in post-industrial HGM. Growth on β-mannans, β(1, 3)-galactans, and microbial β(1, 3)-glucans was not observed, due to an apparent lack of cognate PULs. Most notably, S. copri is unable to grow on starch, due to an incomplete starch utilization system (Sus). Subsequent transcriptional profiling of bellwether Ton-B-dependent transporter-encoding genes revealed that PUL upregulation is rapid and general upon transfer from glucose to plant polysaccharides, reflective of de-repression enabling substrate sensing. Distinct from previous observations of Bacteroides species, we were unable to observe clearly delineated substrate prioritization on a polysaccharide mixture designed to mimic in vitro diverse plant cell wall digesta. Finally, co-culture experiments generally indicated stable co-existence and lack of exclusive competition between S. copri and representative HGM Bacteroides species (Bacteroides thetaiotaomicron and Bacteroides ovatus) on individual polysaccharides, except in cases where corresponding PULs were obviously lacking.
Importance: There is currently a great level of interest in improving the composition and function of the human gut microbiota (HGM) to improve health. The bacterium Segatella copri is prevalent in people who eat plant-rich diets and is therefore associated with a healthy lifestyle. On one hand, our study reveals the specific molecular systems that enable S. copri to proliferate on individual plant polysaccharides. On the other, a growing body of data suggests that the inability of S. copri to grow on starch and animal glycans, which dominate in post-industrial diets, as well as host mucin, contributes strongly to its displacement from the HGM by Bacteroides species, in the absence of direct antagonism.
Keywords: Bacteroidaceae; Bacteroides; Prevotella; Prevotellaceae; Segatella; carbohydrate-active enzymes; dietary fiber; human gut microbiota; plant cell wall; polysaccharide utilization locus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




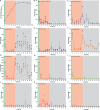
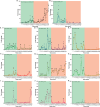

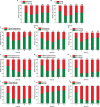
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- MOP-142472,PJ9-185659,PJT-189972/Canadian Government | Canadian Institutes of Health Research (CIHR)
- RGPIN-2018-03892,RGPIN-2024-04318/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
- Banting Postdoctoral Fellowship/Canadian Government | Canadian Institutes of Health Research (CIHR)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

