Focal liver lesions: multiparametric microvasculature characterization via super-resolution ultrasound imaging
- PMID: 39636384
- PMCID: PMC11621259
- DOI: 10.1186/s41747-024-00540-3
Focal liver lesions: multiparametric microvasculature characterization via super-resolution ultrasound imaging
Abstract
Background: Noninvasive and functional imaging of the focal liver lesion (FLL) vasculature at microscopic scales is clinically challenging. We investigated the feasibility of using super-resolution ultrasound (SR-US) imaging for visualizing and quantifying the microvasculature of intraparenchymal FLLs.
Methods: Patients with FLLs between June 2022 and February 2023 were prospectively screened. Following bolus injection of microbubbles at clinical concentration, SR-US was performed using a high frame rate (350-500 Hz) modified ultrasound scanner and a convex array transducer with a central frequency of 3.1 MHz.
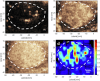
Results: In total, 47 pathologically proven FLLs at a depth of 5.7 ± 1.7 cm (mean ± standard deviation) were included: 30 hepatocellular carcinomas (HCC), 11 liver metastases (LM), and 6 focal nodular hyperplasias (FNH). The smallest detectable vessel size of the hepatic microvasculature was 128.4 ± 18.6 μm (mean ± standard deviation) at a depth of 8 cm. Significant differences were observed among the three types of lesions in terms of pattern categories, vessel density, minimum flow velocity, and perfusion index. We observed higher vessel density for FNH versus liver parenchyma (p < 0.001) as well as fractal dimension and local flow direction entropy value for FNH versus HCC (p = 0.002 and p < 0.001, respectively) and for FNH versus LM (p = 0.006 and p = 0.002, respectively).
Conclusion: Multiparametric SR-US showed that these three pathological types of FLLs have specific microvascular phenotypes. Vessel density, fractal dimension and local flow direction entropy served as valuable parameters in distinguishing between benign and malignant FLLs.
Trial registration: ClinicalTrials.gov (NCT06018142).
Relevance statement: Multiparametric SR-US imaging offers precise morphological and functional assessment of the microvasculature of intraparenchymal focal liver lesions, providing insights into tumor heterogeneity and angiogenesis.
Key points: Super-resolution (SR)-US imaging allowed morphological and functional evaluation of intraparenchymal hepatic lesion microvasculature. Hepatocellular carcinoma, liver metastasis, and focal nodular hyperplasia exhibit distinct microvascular architectures and hemodynamic profiles. Multiparametric microvasculature characterization via SR-US imaging facilitates the differentiation between benign and malignant microvascular phenotypes.
Keywords: Carcinoma (hepatocellular); Focal nodular hyperplasia; Liver neoplasms; Microbubbles; Ultrasonography.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Our study was registered on ClinicalTrials.gov (NCT06018142), and the study protocol was approved by the Institutional Review Board. Consent to participate was obtained from all patients in this study. Consent for publication: Not applicable. Competing interests: The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Figures





References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
