Mechanisms of ferroptotic and non-ferroptotic organ toxicity of chemotherapy: protective and therapeutic effects of ginger, 6-gingerol and zingerone in preclinical studies
- PMID: 39636404
- PMCID: PMC11985630
- DOI: 10.1007/s00210-024-03623-5
Mechanisms of ferroptotic and non-ferroptotic organ toxicity of chemotherapy: protective and therapeutic effects of ginger, 6-gingerol and zingerone in preclinical studies
Abstract
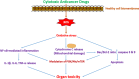
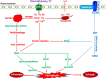
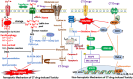
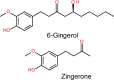
Chemotherapy (CT) is one of the flagship options for the treatment of cancers worldwide. It involves the use of cytotoxic anticancer agents to kill or inhibit the proliferation of cancer cells. However, despite its clinical efficacy, CT triggers side effect toxicities in several organs, which may impact cancer patient's quality of life and treatment outcomes. While the side effect toxicity is consistent with non-ferroptotic mechanisms involving oxidative stress, inflammation, mitochondrial impairment and other aberrant signalling leading to apoptosis and necroptosis, recent studies show that ferroptosis, a non-apoptotic, iron-dependent cell death pathway, is also involved in the pathophysiology of CT organ toxicity. CT provokes organ ferroptosis via system Xc-/GPX-4/GSH/SLC7A11 axis depletion, ferritinophagy, iron overload, lipid peroxidation and upregulation of ferritin-related proteins. Cisplatin (CP) and doxorubicin (DOX) are common CT drugs indicated to induce ferroptosis in vitro and in vivo. Studies have explored natural preventive and therapeutic strategies using ginger rhizome and its major bioactive compounds, 6-gingerol (6G) and zingerone (ZG), to combat mechanisms of CT side effect toxicity. Ginger extract, 6G and ZG mitigate non-ferroptotic oxidative inflammation, apoptosis and mitochondrial dysfunction mechanisms of CT side effect toxicity, but their effects on CT-induced ferroptosis remain unclear. Systematic investigations are, therefore, needed to unfold the roles of ginger, 6G and ZG on ferroptosis involved in CT side effect toxicity, as they are potential natural agents for the prevention of CT toxicity. This review reveals the ferroptotic and non-ferroptotic toxicity mechanisms of CT and the protective mechanisms of ginger, 6G and ZG against CT-induced, ferroptotic and non-ferroptotic organ toxicities.
Keywords: Zingiber officinale; Anticancer drugs; Chemotherapy; Ferroptosis; Ginger rhizome.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Declaration of generative AI and AI-assisted technologies: The authors declare that there was no use of AI technologies in any form during the preparation of this work.
Figures

References
-
- Abdul-Hamid M, Salah M (2016) Intervention of ginger or propolis ameliorates methotrexate-induced ileum toxicity. Toxicol Ind Health 32(2):313–322 - PubMed
-
- Abo Mansour HE, El-Batsh MM, Badawy NS, Mehanna ET, Mesbah NM, Abo-Elmatty DM (2021) Ginger extract loaded into chitosan nanoparticles enhances cytotoxicity and reduces cardiotoxicity of doxorubicin in hepatocellular carcinoma in mice. Nutr Cancer 73(11–12):2347–2362 - PubMed
-
- Abushouk AI, Ismail A, Salem AM, Afifi AM, Abdel-Daim MM (2017) Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed Pharmacother 90:935–946 - PubMed
-
- Adeyemi DH, Obembe OO, Hamed MA, Akhigbe RE (2024) Sodium acetate ameliorates doxorubicin-induced cardiac injury via upregulation of Nrf2/HO-1 signaling and downregulation of NFkB-mediated apoptotic signaling in Wistar rats. Naunyn-Schmiedeberg’s Arch Pharmacol 397(1):423–435 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

