Filbertone-Induced Nrf2 Activation Ameliorates Neuronal Damage via Increasing BDNF Expression
- PMID: 39636503
- PMCID: PMC11621137
- DOI: 10.1007/s11064-024-04290-x
Filbertone-Induced Nrf2 Activation Ameliorates Neuronal Damage via Increasing BDNF Expression
Abstract
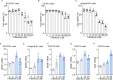
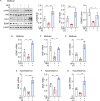
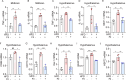
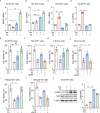
Neurotrophic factors are endogenous proteins that promote the survival of various neuronal cells. Increasing evidence has suggested a key role for brain-derived neurotrophic factor (BDNF) in the dopaminergic neurotoxicity associated with Parkinson's Disease (PD). This study explores the therapeutic potential of filbertone, a bioactive compound found in hazelnuts, in neurodegeneration, focusing on its effects on neurotrophic factors and the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway. In our study, filbertone markedly elevated the expression of neurotrophic factors, including BDNF, Glial cell line-Derived Neurotrophic Factor (GDNF), and Nerve Growth Factor (NGF), in human neuroblastoma SH-SY5Y cells, mouse astrocyte C8-D1A cells, and mouse hypothalamus mHypoE-N1 cells. Moreover, filbertone effectively countered neuroinflammation and reversed the decline in neurotrophic factors and Nrf2 activation induced by a high-fat diet (HFD) in neurodegeneration models. The neuroprotective effects of filbertone were further validated in models of neurotoxicity induced by palmitic acid (PA) and the neurotoxin MPTP/MPP+, where it was observed to counteract PA and MPTP/MPP+-induced decreases in cell viability and neuroinflammation, primarily through the activation of Nrf2 and the subsequent upregulation of BDNF and heme oxygenase-1 expression. Nrf2 deficiency negated the neuroprotective effects of filbertone in MPTP-treated mice. Consequently, our finding suggests that filbertone is a novel therapeutic agent for neurodegenerative diseases, enhancing neuronal resilience through the Nrf2 signaling pathway and upregulation of neurotrophic factors.
Keywords: Filbertone; Heme oxygenase-1; Neuroinflammation; Neurotrophic factors; Nuclear factor erythroid 2-related factor 2; Parkinson’s disease.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical Approval: All experimental procedures were approved by the Ethics Committee of the University of Ulsan University (HTC-22-010) in accordance with the National Institutes of Health guidelines. Efforts were made to minimize animal suffering, and all sample sizes for the assessment parameters were calculated to minimize the number of animals used. Consent to Paricipate: Not applicable. Consent for Publication: Not applicable. Competing Interests: The authors declare no competing interests.
Figures


References
-
- Nussbaum RL, Ellis CE (2003) Alzheimer’s disease and Parkinson’s disease. N Engl J Med 348:1356–1364 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

