Effects of Current Therapies on Disease Progression in Fabry Disease: A Narrative Review for Better Patient Management in Clinical Practice
- PMID: 39636569
- PMCID: PMC11787255
- DOI: 10.1007/s12325-024-03041-2
Effects of Current Therapies on Disease Progression in Fabry Disease: A Narrative Review for Better Patient Management in Clinical Practice
Abstract
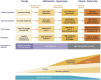
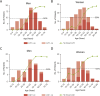
Fabry disease (FD) is a rare lysosomal storage disorder that is characterized by renal, neurological, and cardiovascular dysfunction. Four treatments are currently available for patients with FD; three enzyme replacement therapies (ERTs; agalsidase alfa, agalsidase beta, and pegunigalsidase alfa) and one pharmacological chaperone (migalastat). This review focuses on the evidence for the benefits of ERTs and migalastat, and provides an overview of their impact on disease manifestations and quality of life (QoL). Agalsidase beta is associated with renal, neurological, and cardiovascular benefits, and may prevent renal disease progression. Agalsidase alfa provides stabilizing effects across all main organ systems, although minor sex-specific differences exist in patients with more advanced baseline disease. The benefits of agalsidase alfa and agalsidase beta are similar but depend on the extent of baseline disease. Some data indicate that agalsidase beta may be preferable over the longer term. Both agalsidase alfa and agalsidase beta are associated with improved gastrointestinal and pain symptoms, as well as improved QoL. Patients with advanced end-organ damage tend not to respond as optimally to ERTs as those who initiate ERTs before irreversible organ fibrosis develops, highlighting the need for early treatment initiation. Migalastat, which is only approved for patients with amenable missense gene variants, generally stabilizes renal parameters and provides cardiovascular benefits. Migalastat also improves diarrhea and pain, and stabilizes QoL (although ERT may be more effective for pain management), but the neurological effects of migalastat have not been studied. Real-world data raise concerns about effective in vivo amenability of some genetic variants. Future studies with direct treatment comparisons in patients with FD are needed.
Keywords: Agalsidase alfa; Agalsidase beta; Cardiovascular outcomes; Enzyme replacement therapy; Fabry disease; Neurological outcomes; Pegunigalsidase alfa; Quality of life; Renal outcomes.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interest: Renzo Mignani has received speaker and advisory board honoraria and travel support from Amicus Therapeutics, Chiesi, Takeda, and Sanofi. Maurizio Pieroni has received speaker and advisory board honoraria and travel support from Amicus Therapeutics, Chiesi, Takeda, and Sanofi. Elena Biagini has received speaker and advisory board honoraria and travel support from Amicus Therapeutics, Chiesi, Takeda, Genzyme, and BMS. Vittoria Cianci, Federico Pieruzzi, Antonio Pisani, and Antonino Tuttolomondo have nothing to disclose. Ethical Approval: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Figures

References
-
- National Institute for Health and Care Excellence. Migalastat for treating Fabry disease: highly specialised technologies guidance HST4. 2017. http://www.nice.org.uk/guidance/hst4. Accessed 26 Oct 2022.
-
- Niemann M, Rolfs A, Störk S, et al. Gene mutations versus clinically relevant phenotypes: lyso-Gb3 defines Fabry disease. Circ Cardiovasc Genet. 2014;7(1):8–16. - PubMed
-
- Germain DP, Nicholls K, Giugliani R, et al. Efficacy of the pharmacologic chaperone migalastat in a subset of male patients with the classic phenotype of Fabry disease and migalastat-amenable variants: data from the phase 3 randomized, multicenter, double-blind clinical trial and extension study. Genet Med. 2019;21(9):1987–97. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

