Predictors of Death or Severe Impairment in Neonates With Hypoxic-Ischemic Encephalopathy
- PMID: 39636636
- PMCID: PMC11621987
- DOI: 10.1001/jamanetworkopen.2024.49188
Predictors of Death or Severe Impairment in Neonates With Hypoxic-Ischemic Encephalopathy
Erratum in
-
Errors in Figures.JAMA Netw Open. 2025 Jan 2;8(1):e2459716. doi: 10.1001/jamanetworkopen.2024.59716. JAMA Netw Open. 2025. PMID: 39786781 Free PMC article. No abstract available.
Abstract
Importance: Outcomes after hypoxic-ischemic encephalopathy (HIE) are variable. Predicting death or severe neurodevelopmental impairment (NDI) in affected neonates is crucial for guiding management and parent communication.
Objective: To predict death or severe NDI in neonates who receive hypothermia for HIE.
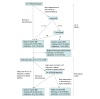
Design, setting, and participants: This prognostic study included participants enrolled in a large US clinical trial conducted in US neonatal intensive care units who were born between January 2017 and October 2019 and followed up to age 2 years. Eligible participants were neonates with moderate-severe HIE born at 36 weeks or more gestation and with 2-year outcome data. Data were analyzed June 2023. External validation was performed with a UK cohort.
Exposure: Clinical, electroencephalography (EEG), and magnetic resonance imaging (MRI) variables were curated and examined at 24 hours and following cooling.
Main outcome and measures: Death or severe NDI at age 2 years. Severe NDI was defined as Bayley Scales of Infant Toddler Development cognitive score below 70, Gross Motor Function Classification System score of 3 or higher, or quadriparesis. Model performance metrics were derived from training, internal, and external validation datasets.
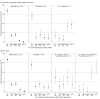
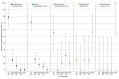
Results: Among 424 neonates (mean [SD] gestational age, 39.1 [1.4] weeks; 192 female [45.3%]; 28 Asian [6.6%], 50 Black [11.8%], 311 White [73.3%]), 105 (24.7%) had severe encephalopathy at enrollment. Overall, 59 (13.9%) died and 46 (10.8%) had severe NDI. In the 24-hour model, the combined presence of 3 clinical characteristics-(1) severely abnormal EEG, (2) pH level of 7.11 or below, and (3) 5-minute Apgar score of 0-had a specificity of 99.6% (95% CI, 97.5%-100%) and a positive predictive value (PPV) of 95.2% (95% CI, 73.2%-99.3%). Validation model metrics were 97.9% (95% CI, 92.7%-99.8%) for internal specificity, with a PPV of 77.8% (95% CI, 43.4%-94.1%), and 97.6% (95% CI, 95.1%-99.0%) for external specificity, with a PPV of 46.2% (95% CI, 23.3%-70.8%). In the postcooling model, specificity for T1, T2, or diffusion-weighted imaging (DWI) abnormality in at least 2 of 3 deep gray regions (ie, thalamus, caudate, putamen and/or globus pallidus) plus a severely abnormal EEG within the first 24 hours was 99.1% (95% CI, 96.8%-99.9%), with a PPV of 91.7% (95% CI, 72.8%-97.8%). Internal specificity in this model was 98.9% (95% CI, 94.1%-100%), with a PPV of 92.9% (95% CI, 64.2%-99.0%); external specificity was 98.6% (95% CI, 96.5%-99.6%), with a PPV of 83.3% (95% CI, 64.1%-93.4%).
Conclusions and relevance: In this prognostic study of neonates with moderate or severe HIE who were treated with therapeutic hypothermia, simple models using readily available clinical, EEG, and MRI results during the hospital admission had high specificity and PPV for death or severe NDI at age 2 years.
Conflict of interest statement
Figures
References
-
- Wu Y. Clinical features, diagnosis, and treatment of neonatal encephalopathy. UpToDate. Updated January 30, 2024. Accessed July 29, 2024. https://www.uptodate.com/contents/clinical-features-diagnosis-and-treatm...
-
- Juul SE, Voldal E, Comstock BA, et al. ; HEAL consortium . Association of high-dose erythropoietin with circulating biomarkers and neurodevelopmental outcomes among neonates with hypoxic ischemic encephalopathy: a secondary analysis of the HEAL randomized clinical trial. JAMA Netw Open. 2023;6(7):e2322131. doi:10.1001/jamanetworkopen.2023.22131 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

