Cardiac Events and Survival in Patients With EGFR-Mutant Non-Small Cell Lung Cancer Treated With Osimertinib
- PMID: 39636639
- PMCID: PMC11621985
- DOI: 10.1001/jamanetworkopen.2024.48364
Cardiac Events and Survival in Patients With EGFR-Mutant Non-Small Cell Lung Cancer Treated With Osimertinib
Abstract
Importance: Although it has been reported that osimertinib mesylate provides better survival benefits compared with first- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs), it remains unclear whether osimertinib is associated with more cancer therapy-related cardiac events (CTRCEs) compared with other EGFR TKIs, as does the extent of the association these adverse effects may have with overall survival. This issue is particularly critical due to the high prevalence of EGFR variants within Asian populations, including that of Taiwan.
Objective: To compare CTRCEs and their association with survival in patients treated with osimertinib vs other EGFR TKIs.
Design, setting, and participants: This cohort study was conducted at the National Cheng Kung University Hospital, a college hospital and tertiary academic referral center in Taiwan. The median follow-up duration was 23.2 (IQR, 15.2-31.5) months. A total of 401 patients with EGFR-mutant non-small cell lung cancer (NSCLC) beginning treatment with EGFR TKIs from September 1, 2019, to July 31, 2022, were retrospectively analyzed. CTRCEs included newly emerging arrhythmias, valvular heart diseases (moderate and more), myocardial infarction, and heart failure and were analyzed after adjusting for age, sex, smoking, alcohol consumption, body mass index, cardiovascular comorbidities, thoracic radiotherapy, and cardiovascular medications. Follow-up was completed January 31, 2024.
Exposure: Osimertinib.
Main outcomes and measures: The Cox proportional hazards model was used to estimate CTRCEs in patients treated with osimertinib or other EGFR TKIs. Considering that death can lower the incidence of CTRCEs, the competing risk method was used to calculate CTRCEs after adjusting for potential confounders. Multivariable Cox proportional hazard regression analysis for overall survival was used to explore whether CTRCEs were independently associated with overall survival.
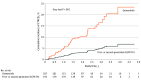
Results: Among the 401 patients (253 [63.1%] female; mean [SD] age, 69.2 [11.3] years), 195 (48.6%) treated with osimertinib were matched with 206 (51.4%) treated with other EGFR TKIs. Occurrence of CTRCEs in patients receiving osimertinib was significantly higher compared with patients treated with other EGFR TKIs (29 [14.9%] vs 9 [4.4%]; hazard ratio [HR], 3.37; 95% CI, 1.56-7.26; P = .002). After adjustment for relevant cardiovascular risk factors, the HR of CTRCEs was significantly higher in the group treated with osimertinib (adjusted subdistribution HR, 4.00; 95% CI, 1.81-8.85; P < .001). In addition, CTRCEs were independently associated with overall survival (HR, 4.02; 95% CI, 2.44-6.63; P < .001).
Conclusions and relevance: In this cohort study of patients with EGFR-mutant NSCLC, osimertinib was associated with a higher incidence of CTRCEs compared with other EGFR TKIs; CTRCEs were independently associated with overall survival. These findings highlight the need for ongoing cardiac monitoring in these patients, regardless of preexisting cardiac risk factors.
Conflict of interest statement
Figures
Comment in
References
-
- Goldstraw P, Chansky K, Crowley J, et al. ; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions . The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(1):39-51. doi: 10.1016/j.jtho.2015.09.009 - DOI - PubMed
-
- Rosell R, Carcereny E, Gervais R, et al. ; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica . Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation–positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi: 10.1016/S1470-2045(11)70393-X - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

