Dynamic tracking of native precursors in adult mice
- PMID: 39636670
- PMCID: PMC11620740
- DOI: 10.7554/eLife.97504
Dynamic tracking of native precursors in adult mice
Abstract
Hematopoietic dysfunction has been associated with a reduction in the number of active precursors. However, precursor quantification at homeostasis and under diseased conditions is constrained by the scarcity of available methods. To address this issue, we optimized a method for quantifying a wide range of hematopoietic precursors. Assuming the random induction of a stable label in precursors following a binomial distribution, estimates depend on the inverse correlation between precursor numbers and the variance of precursor labeling among independent samples. Experimentally validated to cover the full dynamic range of hematopoietic precursors in mice (1-105), we utilized this approach to demonstrate that thousands of precursors, which emerge after modest expansion during fetal-to-adult transition, contribute to native and perturbed hematopoiesis. We further estimated the number of precursors in a mouse model of Fanconi Anemia, showcasing how repopulation deficits can be classified as autologous (cell proliferation) and non-autologous (lack of precursor). Our results support an accessible and reliable approach for precursor quantification, emphasizing the contemporary perspective that native hematopoiesis is highly polyclonal.
Keywords: Fanconi Anemia; clonal diversity; hematopoiesis; mouse; regenerative medicine; stem cells.
© 2024, Liu et al.
Conflict of interest statement
SL, SA, HZ, JE, SJ, GJ, TM, LP, HL, PK No competing interests declared
Figures
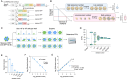









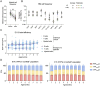



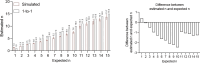
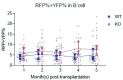

Update of
-
Dynamic Tracking of Native Precursors in Adult Mice.bioRxiv [Preprint]. 2024 Sep 5:2024.04.02.587737. doi: 10.1101/2024.04.02.587737. bioRxiv. 2024. Update in: Elife. 2024 Dec 05;13:RP97504. doi: 10.7554/eLife.97504. PMID: 38617223 Free PMC article. Updated. Preprint.
Similar articles
-
Dynamic Tracking of Native Precursors in Adult Mice.bioRxiv [Preprint]. 2024 Sep 5:2024.04.02.587737. doi: 10.1101/2024.04.02.587737. bioRxiv. 2024. Update in: Elife. 2024 Dec 05;13:RP97504. doi: 10.7554/eLife.97504. PMID: 38617223 Free PMC article. Updated. Preprint.
-
Fetal origins of hematopoietic failure in a murine model of Fanconi anemia.Blood. 2013 Mar 14;121(11):2008-12. doi: 10.1182/blood-2012-06-439679. Epub 2013 Jan 11. Blood. 2013. PMID: 23315168 Free PMC article.
-
Hoxb4-deficient mice undergo normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells.Blood. 2004 Jun 1;103(11):4126-33. doi: 10.1182/blood-2003-10-3557. Epub 2004 Feb 12. Blood. 2004. PMID: 14962901
-
Characteristics of the hematopoietic stem cell compartment in adult mice.Int J Cell Cloning. 1992 Sep;10(5):277-85. doi: 10.1002/stem.5530100505. Int J Cell Cloning. 1992. PMID: 1453014 Review.
-
Why hematopoietic stem cells fail in Fanconi anemia: Mechanisms and models.Bioessays. 2025 Jan;47(1):e2400191. doi: 10.1002/bies.202400191. Epub 2024 Oct 25. Bioessays. 2025. PMID: 39460396 Review.
References
-
- Bowling S, Sritharan D, Osorio FG, Nguyen M, Cheung P, Rodriguez-Fraticelli A, Patel S, Yuan WC, Fujiwara Y, Li BE, Orkin SH, Hormoz S, Camargo FD. An Engineered CRISPR-Cas9 Mouse Line for Simultaneous Readout of Lineage Histories and Gene Expression Profiles in Single Cells. Cell. 2020;181:1410–1422. doi: 10.1016/j.cell.2020.04.048. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

