Self-Assembly and Biological Properties of Highly Fluorinated Oligonucleotide Amphiphiles
- PMID: 39636686
- PMCID: PMC11811686
- DOI: 10.1002/anie.202419996
Self-Assembly and Biological Properties of Highly Fluorinated Oligonucleotide Amphiphiles
Abstract
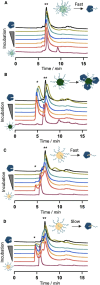
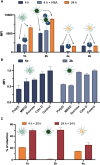
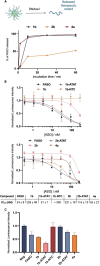
Nucleic acids, used as therapeutics to silence disease-related genes, offer significant advantages over small molecule drugs: they provide high specificity, the ability to target "undruggable" molecules, and adaptability to a wide range of disease phenotypes. However, their instability in biological media, as well their rapid clearance from the organism limit their applicability, necessitating the use of nanocarriers to overcome these challenges. Among these strategies, spherical nucleic acids (SNA)-composed of a densely packed corona of oligonucleotides around a nanoparticle-have emerged as a powerful tool, in particular when self-assembled from DNA amphiphiles. This non-covalent strategy however has caveats, especially when it comes to stability in complex biological media, where these SNAs disassemble in contact to serum proteins. Here, we developed highly fluorinated DNA amphiphiles that readily self-assemble into SNAs and have tunable stability profiles in biological media. They are made of branched fluorinated moieties with potentially improved biodegradability as compared to their linear counterparts. Depending on the number of fluorophilic interactions, the self-assembled SNAs can have excellent serum stabilities-up to days-and readily deliver nucleic acid therapeutics for gene silencing applications. These systems show great potential as promising candidates for nucleic acid-based therapies.
Keywords: delivery; fluorine; gene silencing; oligonucleotides; self-assembly.
© 2024 The Author(s). Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kulkarni J. A., Witzigmann D., Thomson S. B., Chen S., Leavitt B. R., Cullis P. R., van der Meel R., Nat. Nanotechnol. 2021, 16, 630–643. - PubMed
-
- Bauhuber S., Hozsa C., Breunig M., Göpferich A., Adv. Mater. 2009, 21, 3286–3306. - PubMed
-
- Kulkarni J. A., Witzigmann D., Chen S., Cullis P. R., van der Meel R., Acc. Chem. Res. 2019, 52, 2435–2444. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

