Safety, effectiveness and immunogenicity of heterologous mRNA-1273 boost after prime with Ad26.COV2.S among healthcare workers in South Africa: The single-arm, open-label, phase 3 SHERPA study
- PMID: 39636838
- PMCID: PMC11620404
- DOI: 10.1371/journal.pgph.0003260
Safety, effectiveness and immunogenicity of heterologous mRNA-1273 boost after prime with Ad26.COV2.S among healthcare workers in South Africa: The single-arm, open-label, phase 3 SHERPA study
Abstract
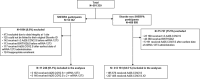
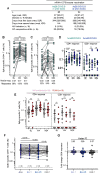
Limited studies have been conducted on the safety and effectiveness of heterologous COVID-19 vaccine boosting in lower income settings, especially those with high-HIV prevalence., The Sisonke Heterologous mRNA-1273 boost after prime with Ad26.COV2.S (SHERPA) trial evaluated a mRNA-1273 boost after Ad26.COV2.S priming in South Africa. SHERPA was a single-arm, open-label, phase 3 study nested in the Sisonke implementation trial of 500000 healthcare workers (HCWs). Sisonke participants were offered mRNA-1273 boosters between May and November 2022, when Omicron sub-lineages were circulating. Adverse events (AE) were self-reported, and co-primary endpoints (SARS-CoV-2 infections and COVID-19 hospitalizations or deaths) were collected through national databases. We used Cox regression models with booster status as a time-varying covariate to determine the relative vaccine effectiveness (rVE) of the mRNA-1273 booster among SHERPA versus unboosted Sisonke participants. Of 11248 SHERPA participants in the rVE analysis cohort (79.3% female, median age 41), 45.4% had received one and 54.6% two Ad26.COV2.S doses. Self-reported comorbidities included HIV (18.7%), hypertension (12.9%) and diabetes (4.6%). In multivariable analysis including 413161 unboosted Sisonke participants, rVE of the booster was 59% (95%CI 29-76%) against SARS-CoV-2 infection: 77% (95%CI 9-94%) in the one-Ad26.COV2.S dose group and 52% (95%CI 13-73%) in the two-dose group. Severe COVID-19 was identified in 148 unboosted Sisonke participants, and only one SHERPA participant with severe HIV-related immunosuppression. Of 11798 participants in the safety analysis, 228 (1.9%) participants reported 575 reactogenicity events within 7 days of the booster (most commonly injection site pain, malaise, myalgia, swelling, induration and fever). More reactogenicity events were reported among those with prior SARS-CoV-2 infections (adjusted odds ratio [aOR] 2.03, 95%CI 1.59-2.59) and less among people living with HIV (PLWH) (aOR 0.49, 95%CI 0.34-0.69). There were 115 unsolicited adverse events (AEs) within 28 days of vaccination. No related serious AEs were reported. In an immunogenicity sub-study, mRNA-1273 increased binding and neutralizing antibody titres and spike-specific T-cell responses 4 weeks after boosting regardless of the number of prior Ad26.COV2.S doses, or HIV status, and generated Omicron spike-specific cross-reactive responses. mRNA-1273 boosters after one or two Ad26.COV2.S doses were well-tolerated, safe and effective against Omicron SARS-CoV-2 infections among HCWs and PLWH. Trial registration: The SHERPA study is registered in the Pan African Clinical Trials Registry (PACTR): PACTR202310615330649 and the South African National Clinical Trial Registry (SANCTR): DOH-27-052022-5778.
Copyright: © 2024 Garrett et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
KA and BL are employees of Moderna, Inc. and may hold stock/stock options in the company. The other authors declare no conflict of interests.
Figures

References
-
- Bekker L-G, Garrett N, Goga A, Fairall L, Reddy T, Yende-Zuma N, et al.. Effectiveness of the Ad26.COV2.S vaccine in health-care workers in South Africa (the Sisonke study): results from a single-arm, open-label, phase 3B, implementation study. Lancet 2022; 399(10330): 1141–53. doi: 10.1016/S0140-6736(22)00007-1 - DOI - PMC - PubMed
-
- Hardt K, Vandebosch A, Sadoff J, Le Gars M, Truyers C, Lowson D, et al.. Efficacy, safety, and immunogenicity of a booster regimen of Ad26.COV2.S vaccine against COVID-19 (ENSEMBLE2): results of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect Dis 2022; 22(12): 1703–15. doi: 10.1016/S1473-3099(22)00506-0 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
