Intricate ribosome composition and translational reprogramming in epithelial-mesenchymal transition
- PMID: 39636864
- PMCID: PMC11648652
- DOI: 10.1073/pnas.2408114121
Intricate ribosome composition and translational reprogramming in epithelial-mesenchymal transition
Abstract
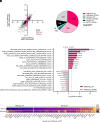
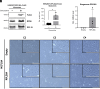
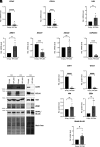
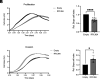
Epithelial-mesenchymal transition (EMT) involves profound changes in cell morphology, driven by transcriptional and epigenetic reprogramming. However, evidence suggests that translation and ribosome composition also play key roles in establishing pathophysiological phenotypes. Using genome-wide analyses, we reported significant rearrangement of the translational landscape and machinery during EMT. Specifically, a cell line overexpressing the EMT transcription factor ZEB1 displayed alterations in translational reprogramming and fidelity. Furthermore, using riboproteomics, we unveiled an increased level of the ribosomal protein RPL36A in mesenchymal ribosomes, indicating precise tuning of ribosome composition. Remarkably, RPL36A overexpression alone was sufficient to trigger the acquisition of mesenchymal features, including a switch in the molecular pattern, cell morphology, and behavior, demonstrating its pivotal role in EMT. These findings underline the importance of translational reprogramming and fine-tuning of ribosome composition in EMT.
Keywords: EMT; RPL36A; ZEB1; ribosome; translation.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

