Programming tissue-sensing T cells that deliver therapies to the brain
- PMID: 39636984
- PMCID: PMC11900893
- DOI: 10.1126/science.adl4237
Programming tissue-sensing T cells that deliver therapies to the brain
Abstract
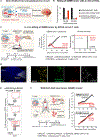
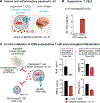
To engineer cells that can specifically target the central nervous system (CNS), we identified extracellular CNS-specific antigens, including components of the CNS extracellular matrix and surface molecules expressed on neurons or glial cells. Synthetic Notch receptors engineered to detect these antigens were used to program T cells to induce the expression of diverse payloads only in the brain. CNS-targeted T cells that induced chimeric antigen receptor expression efficiently cleared primary and secondary brain tumors without harming cross-reactive cells outside of the brain. Conversely, CNS-targeted cells that locally delivered the immunosuppressive cytokine interleukin-10 ameliorated symptoms in a mouse model of neuroinflammation. Tissue-sensing cells represent a strategy for addressing diverse disorders in an anatomically targeted manner.
Conflict of interest statement
The remaining authors declare no competing interests.
Figures



Comment in
-
Synthetic gene circuits drive disease-fighting T cells.Science. 2024 Dec 6;386(6726):1094-1095. doi: 10.1126/science.adt9921. Epub 2024 Dec 5. Science. 2024. PMID: 39637005
-
Engineered T cells traverse new terrain.Nat Rev Drug Discov. 2025 Feb;24(2):88. doi: 10.1038/d41573-025-00002-4. Nat Rev Drug Discov. 2025. PMID: 39779877 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

