Functional investigation suggests CNTNAP5 involvement in glaucomatous neurodegeneration obtained from a GWAS in primary angle closure glaucoma
- PMID: 39637236
- PMCID: PMC11651621
- DOI: 10.1371/journal.pgen.1011502
Functional investigation suggests CNTNAP5 involvement in glaucomatous neurodegeneration obtained from a GWAS in primary angle closure glaucoma
Abstract
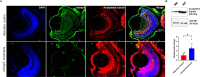
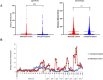
Primary angle closure glaucoma (PACG) affects more than 20 million people worldwide, with an increased prevalence in south-east Asia. In a prior haplotype-based Genome Wide Association Study (GWAS), we identified a novel CNTNAP5 genic region, significantly associated with PACG. In the current study, we have extended our perception of CNTNAP5 involvement in glaucomatous neurodegeneration in a zebrafish model, through investigating phenotypic consequences pertinent to retinal degeneration upon knockdown of cntnap5 by translation-blocking morpholinos. While cntnap5 knockdown was successfully validated using an antibody, immunofluorescence followed by western blot analyses in cntnap5-morphant (MO) zebrafish revealed increased expression of acetylated tubulin indicative of perturbed cytoarchitecture of retinal layers. Moreover, significant loss of Nissl substance is observed in the neuro-retinal layers of cntnap5-MO zebrafish eye, indicating neurodegeneration. Additionally, in spontaneous movement behavioural analysis, cntnap5-MO zebrafish have a significantly lower average distance traversed in light phase compared to mismatch-controls, whereas no significant difference was observed in the dark phase, corroborating with vision loss in the cntnap5-MO zebrafish. This study provides the first direct functional evidence of a putative role of CNTNAP5 in visual neurodegeneration.
Copyright: © 2024 Chakraborty et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

