Disentangling the effects of intermittent faecal shedding and imperfect test sensitivity on the microscopy-based detection of gut parasites in stool samples
- PMID: 39637237
- PMCID: PMC11717355
- DOI: 10.1371/journal.pntd.0012719
Disentangling the effects of intermittent faecal shedding and imperfect test sensitivity on the microscopy-based detection of gut parasites in stool samples
Abstract
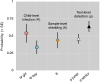
Background: Gut-parasite transmission often involves faecal shedding, and detecting parasites in stool samples remains the cornerstone of diagnosis. However, not all samples drawn from infected hosts contain parasites (because of intermittent shedding), and no test can detect the target parasites in 100% of parasite-bearing samples (because of imperfect sensitivity). Disentangling the effects of intermittent shedding and imperfect sensitivity on pathogen detection would help us better understand transmission dynamics, disease epidemiology, and diagnostic-test performance. Using paediatric Giardia infections as a case-study, here we illustrate a hierarchical-modelling approach to separately estimating the probabilities of host-level infection ([Formula: see text]); stool-sample-level shedding, given infection ([Formula: see text]); and test-level detection, given infection and shedding ([Formula: see text]).
Methods/findings: We collected 1-3 stool samples, in consecutive weeks, from 276 children. Samples (413 overall) were independently examined, via standard sedimentation/optical microscopy, by a senior parasitologist and a junior, trained student (826 tests overall). Using replicate test results and multilevel hierarchical models, we estimated per-sample Giardia shedding probability at [Formula: see text] and observer-specific test sensitivities at [Formula: see text] and [Formula: see text]. Gender-specific infection-frequency estimates were [Formula: see text] and [Formula: see text]. Had we used a (hypothetical) Perfect Test with 100% narrow-sense sensitivity ([Formula: see text]), the average probability of detecting Giardia in a sample drawn from an infected child ([Formula: see text]) would have been [Formula: see text]. Because no test can be >100% sensitive, [Formula: see text] (which measures clinical sensitivity) can only be brought above ~ 0.44 by tinkering with the availability of Giardia in stool samples (i.e., [Formula: see text]); for example, drawing-and-pooling 3 replicate samples would yield [Formula: see text].
Conclusions: By allowing separate estimation (and modelling) of pathogen-shedding probabilities, the approach we illustrate provides a means to study pathogen transmission cycles and dynamics in unprecedented detail. Separate estimation (and modelling) of true test sensitivity, moreover, may cast new light on the performance of diagnostic tests and procedures, whether novel or routine-practice.
Copyright: © 2024 Ferreira-Sá et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- World Health Organization. Diarrhoeal disease. WHO; 2024. Available from https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease.
-
- World Health Organization. Bench aids for the diagnosis of intestinal parasites, 2nd ed. Geneva: WHO; 2019.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

