Receptor binding and structural basis of raccoon dog ACE2 binding to SARS-CoV-2 prototype and its variants
- PMID: 39637248
- PMCID: PMC11620640
- DOI: 10.1371/journal.ppat.1012713
Receptor binding and structural basis of raccoon dog ACE2 binding to SARS-CoV-2 prototype and its variants
Abstract
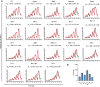
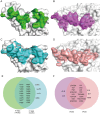
Raccoon dog was proposed as a potential host of SARS-CoV-2, but no evidence support such a notion. In our study, we investigated the binding affinities of raccoon dog ACE2 (rdACE2) to the spike (S) protein receptor binding domain (RBD) of SARS-CoV-2 prototype (PT) and its variants. It revealed that the binding affinities of RBD from SARS-CoV-2 variants were generally lower than that of the PT RBD. Through structural and functional analyses, we found amino acids H34 and M82 play pivotal roles in maintaining the binding affinity of ACE2 to different SARS-CoV-2 sub-variants. These results suggest that raccoon dogs exhibit lower susceptibility to SARS-CoV-2 compared to those animal species with a high prevalence of SARS-CoV-2 transmission.
Copyright: © 2024 Luo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Garbino J, Crespo S, Aubert JD, Rochat T, Ninet B, Deffernez C, et al. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (Non-SARS)-related human coronavirus infection. Clin Infect Dis. 2006;43(8):1009–15. Epub 2006/09/20. doi: 10.1086/507898 ; PubMed Central PMCID: PMC7107919. - DOI - PMC - PubMed
-
- Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884–95. Epub 2004/12/23. doi: 10.1128/JVI.79.2.884-895.2005 ; PubMed Central PMCID: PMC538593. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

