SENP3-FIS1 axis promotes mitophagy and cell survival under hypoxia
- PMID: 39638786
- PMCID: PMC11621581
- DOI: 10.1038/s41419-024-07271-8
SENP3-FIS1 axis promotes mitophagy and cell survival under hypoxia
Abstract
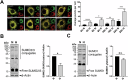
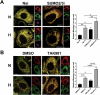
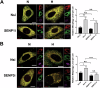
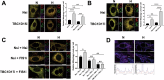
SUMOylation, the covalent attachment of the small ubiquitin-like modifier (SUMO) to target proteins, and its reversal, deSUMOylation by SUMO proteases like Sentrin-specific proteases (SENPs), are crucial for initiating cellular responses to hypoxia. However, their roles in subsequent adaptation processes to hypoxia such as mitochondrial autophagy (mitophagy) remain unexplored. Here, we show that general SUMOylation, particularly SUMO2/3 modification, suppresses mitophagy under both normoxia and hypoxia. Furthermore, we identify deSUMO2/3-ylation enzyme SENP3 and mitochondrial Fission protein 1 (FIS1) as key players in hypoxia-induced mitophagy (HIM), with SUMOylatable FIS1 acting as a crucial regulator for SENP3-mediated HIM regulation. Interestingly, we find that hypoxia promotes FIS1 SUMO2/3-ylation and triggers an interaction between SUMOylatable FIS1 and Rab GTPase-activating protein Tre-2/Bub2/Cdc16 domain 1 family member 17 (TBC1D17), which in turn suppresses HIM. Therefore, we propose a novel SUMOylation-dependent pathway where the SENP3-FIS1 axis promotes HIM, with TBC1D17 acting as a fine-tuning regulator. Importantly, the SENP3-FIS1 axis plays a protective role against hypoxia-induced cell death, highlighting its physiological significance, and hypoxia-inducible FIS1-TBC1D17 interaction is detectable in primary glioma stem cell-like (GSC) cultures derived from glioblastoma patients, suggesting its disease relevance. Our findings not only provide new insights into SUMOylation/deSUMOylation regulation of HIM but also suggest the potential of targeting this pathway to enhance cellular resilience under hypoxic stress.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods were conducted in accordance with relevant guidelines and regulations, including the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all human subjects. Ethics approval for deriving primary GSC cultures was granted by the Yorkshire & The Humber - Leeds East Research Ethics Committee (IRB protocol 11-YH-0319/STH15598). Ethics approval for deriving primary renal cell cultures was obtained from the Research Ethical Committee (REC: 20SW0193) as part of the Ex Vivo DEtermiNed Cancer Therapy (EVIDENT) trial (NCT05231655).
Figures






References
-
- Russell OM, Lightowlers RN, Turnbull DM. Applying the Airbrakes: Treating Mitochondrial Disease with Hypoxia. Mol Cell. 2016;62:5–6. - PubMed
-
- Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochimica et biophysica acta. 2010;1797:1171–7. - PubMed
-
- Wang CY, She JX. SUMO4 and its role in type 1 diabetes pathogenesis. Diabetes/Metab Res Rev. 2008;24:93–102. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

