Identification of AChE targeted therapeutic compounds for Alzheimer's disease: an in-silico study with DFT integration
- PMID: 39638823
- PMCID: PMC11621528
- DOI: 10.1038/s41598-024-81285-2
Identification of AChE targeted therapeutic compounds for Alzheimer's disease: an in-silico study with DFT integration
Abstract
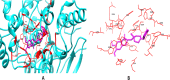
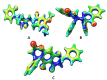
Alzheimer's disease (AD) is a progressive neurodegenerative condition marked by cognitive deterioration and changes in behavior. Acetylcholinesterase (AChE), which hydrolyzes acetylcholine, is a key drug target for treating AD. This research aimed to identify new AChE inhibitors using the IMPPAT database. We used known drugs as a basis to search for similar chemicals in the IMPPAT database and created a library of 127 plant-based compounds. Initial screening of these compounds was performed using molecular docking, followed by an analysis of their drug-likeness and ADMET properties. Compounds with favorable properties underwent density functional theory (DFT) calculations to assess their electronic properties such as HOMO-LUMO gap, electron density, and molecular orbital distribution. These descriptors provided insights into each compound's reactivity, stability, and binding potential with AChE. Promising candidates were further evaluated through molecular dynamics (MD) simulations over 100 ns and MMPBSA analysis for the last 30 ns. Two compounds, Biflavanone (IMPHY013027) with a binding free energy of - 130.394 kcal/mol and Calomelanol J (IMPHY007737) with - 107.908 kcal/mol, demonstrated strong binding affinities compared to the reference molecule HOR, which has a binding free energy of - 105.132 kcal/mol. These compounds exhibited promising drug-ability profiles in both molecular docking and MD simulations, indicating their potential as novel AChE inhibitors for AD treatment. However, further experimental validation is necessary to verify their effectiveness and safety.
Keywords: Alzheimer’s disease; Chemical similarity search; DFT; MD simulation; Molecular docking.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

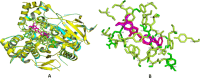






Similar articles
-
Structural Bioinformatics Applied to Acetylcholinesterase Enzyme Inhibition.Int J Mol Sci. 2025 Apr 17;26(8):3781. doi: 10.3390/ijms26083781. Int J Mol Sci. 2025. PMID: 40332446 Free PMC article. Review.
-
Computational Investigation of Novel Compounds as Dual Inhibitors of AChE and GSK-3β for the Treatment of Alzheimer's Disease.Curr Top Med Chem. 2024;24(19):1738-1753. doi: 10.2174/0115680266295740240602122613. Curr Top Med Chem. 2024. PMID: 38859777
-
Lead Identification Through In Silico Studies: Targeting Acetylcholinesterase Enzyme Against Alzheimer's Disease.Cent Nerv Syst Agents Med Chem. 2024;24(2):219-242. doi: 10.2174/0118715249268585240107184956. Cent Nerv Syst Agents Med Chem. 2024. PMID: 38288823
-
Structure-based virtual screening of Trachyspermum ammi metabolites targeting acetylcholinesterase for Alzheimer's disease treatment.PLoS One. 2024 Dec 17;19(12):e0311401. doi: 10.1371/journal.pone.0311401. eCollection 2024. PLoS One. 2024. Retraction in: PLoS One. 2025 May 8;20(5):e0324094. doi: 10.1371/journal.pone.0324094. PMID: 39689077 Free PMC article. Retracted.
-
A Review on Recent Approaches on Molecular Docking Studies of Novel Compounds Targeting Acetylcholinesterase in Alzheimer Disease.Molecules. 2023 Jan 21;28(3):1084. doi: 10.3390/molecules28031084. Molecules. 2023. PMID: 36770750 Free PMC article. Review.
Cited by
-
Revealing Lingonberry's Neuroprotective Potential in Alzheimer's Disease Through Network Pharmacology and Molecular Docking.Int J Mol Sci. 2025 Mar 6;26(5):2363. doi: 10.3390/ijms26052363. Int J Mol Sci. 2025. PMID: 40076984 Free PMC article.
-
Integrated in silico identification of cholinesterase inhibitors from Nyctanthes arbor-tristis.PLoS One. 2025 Jul 23;20(7):e0328457. doi: 10.1371/journal.pone.0328457. eCollection 2025. PLoS One. 2025. PMID: 40700397 Free PMC article.
-
Ameliorative role of Polyscias fruticosa leaf extract in aluminum chloride-induced neurotoxicity flies possibly mediated by N-methyl-D-aspartate receptor antagonistic and anticholinesterase active compounds.J Nat Med. 2025 Sep;79(5):1167-1187. doi: 10.1007/s11418-025-01928-0. Epub 2025 Jul 11. J Nat Med. 2025. PMID: 40646316
-
Structural Bioinformatics Applied to Acetylcholinesterase Enzyme Inhibition.Int J Mol Sci. 2025 Apr 17;26(8):3781. doi: 10.3390/ijms26083781. Int J Mol Sci. 2025. PMID: 40332446 Free PMC article. Review.
-
Mechanistic study on ligustilide modulation of the TLR4/NF-κB pathway in ameliorating Scopolamine-Induced cognitive impairment.Metab Brain Dis. 2025 Aug 15;40(6):248. doi: 10.1007/s11011-025-01672-0. Metab Brain Dis. 2025. PMID: 40815396
References
-
- Rajesh, V. et al. Memory enhancing activity of Lawsonia inermis Linn. Leaves against scopolamine induced memory impairment in Swiss albino mice. Orient. Pharm. Exp. Med.17 (2), 127–142 (2017).
-
- Patterson, C. The State of the Art of Dementia Research: New Frontiers. An Analysis of Prevalence, Incidence, Cost and Trends (Alzheimer’s Disease International, 2018).
-
- Dementia-in-India-. https://dementiacarenotes.in/dcnfiles/Dementia-in-India-2020.pdf (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

