The activation of the piriform cortex to lateral septum pathway during chronic social defeat stress is crucial for the induction of behavioral disturbance in mice
- PMID: 39638863
- PMCID: PMC11914691
- DOI: 10.1038/s41386-024-02034-7
The activation of the piriform cortex to lateral septum pathway during chronic social defeat stress is crucial for the induction of behavioral disturbance in mice
Abstract
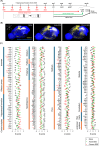
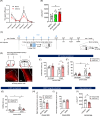
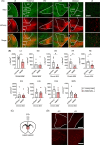
Chronic stress induces neural dysfunctions and risks mental illnesses. Clinical and preclinical studies have established the roles of brain regions underlying emotional and cognitive functions in stress and depression. However, neural pathways to perceive sensory stimuli as stress to cause behavioral disturbance remain unknown. Using whole-brain imaging of Arc-dVenus neuronal response reporter mice and machine learning analysis, here we unbiasedly demonstrated different patterns of contribution of widely distributed brain regions to neural responses to acute and chronic social defeat stress (SDS). Among these brain regions, multiple sensory cortices, especially the piriform (olfactory) cortex, primarily contributed to classifying neural responses to chronic SDS. Indeed, SDS-induced activation of the piriform cortex was augmented with repetition of SDS, accompanied by impaired odor discrimination. Axonal tracing and chemogenetic manipulation showed that excitatory neurons in the piriform cortex directly project to the lateral septum and activate it in response to chronic SDS, thereby inducing behavioral disturbance. These results pave the way for identifying a spatially defined sequence of neural consequences of stress and the roles of sensory pathways in perceiving chronic stress in mental illness pathology.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Social defeat drives hyperexcitation of the piriform cortex to induce learning and memory impairment but not mood-related disorders in mice.Transl Psychiatry. 2022 Sep 10;12(1):380. doi: 10.1038/s41398-022-02151-1. Transl Psychiatry. 2022. PMID: 36088395 Free PMC article.
-
Chronic Social Defeat Stress Induces Pathway-Specific Adaptations at Lateral Habenula Neuronal Outputs.J Neurosci. 2024 Sep 25;44(39):e2082232024. doi: 10.1523/JNEUROSCI.2082-23.2024. J Neurosci. 2024. PMID: 39164106 Free PMC article.
-
Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice.Mol Psychiatry. 2018 Aug;23(8):1717-1730. doi: 10.1038/mp.2017.177. Epub 2017 Sep 19. Mol Psychiatry. 2018. PMID: 28924188
-
The Olfactory Mosaic: Bringing an Olfactory Network Together for Odor Perception.Perception. 2017 Mar-Apr;46(3-4):320-332. doi: 10.1177/0301006616663216. Epub 2016 Sep 29. Perception. 2017. PMID: 27687814 Free PMC article. Review.
-
Neural mechanisms underlying adaptive and maladaptive consequences of stress: Roles of dopaminergic and inflammatory responses.Psychiatry Clin Neurosci. 2019 Nov;73(11):669-675. doi: 10.1111/pcn.12901. Epub 2019 Jul 1. Psychiatry Clin Neurosci. 2019. PMID: 31215710 Review.
Cited by
-
Somatostatin-expressing interneurons of prefrontal cortex modulate social deficits in the Magel2 mouse model of autism.Mol Autism. 2025 Mar 11;16(1):18. doi: 10.1186/s13229-025-00653-5. Mol Autism. 2025. PMID: 40069835 Free PMC article.
References
-
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. - PubMed
-
- Sanacora G, Yan Z, Popoli M. The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat Rev Neurosci. 2022;23:86–103. - PubMed
MeSH terms
Grants and funding
- JP21H04812/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- JP24K22086/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- JP22K06859/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- JPMJMS239F/MEXT | Japan Science and Technology Agency (JST)
- JP24zf0127010/Japan Agency for Medical Research and Development (AMED)
- JP24zf0127012/Japan Agency for Medical Research and Development (AMED)
- JP24wm0425001/Japan Agency for Medical Research and Development (AMED)
- JP21zf0127004/Japan Agency for Medical Research and Development (AMED)
- JP24wm0625117/Japan Agency for Medical Research and Development (AMED)
- JP24gm6310028/Japan Agency for Medical Research and Development (AMED)
- JP23KJ1550/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP21J01369/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24K18224/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Medical

