Single versus tandem autologous stem cell transplantation in newly diagnosed multiple myeloma
- PMID: 39638882
- PMCID: PMC11893441
- DOI: 10.1038/s41409-024-02490-1
Single versus tandem autologous stem cell transplantation in newly diagnosed multiple myeloma
Abstract
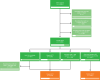
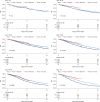
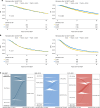
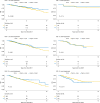
Identifying patients who may benefit from autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma is crucial, especially in the era of effective induction and consolidation strategies. We analyzed data from 12763 patients enrolled in the German Registry for Hematopoietic Stem Cell Transplantation and Cell Therapy (DRST), distinguishing those who underwent single (n = 8736) or tandem ASCT (n = 4027) from 1998 to 2021. Our findings show that the median age at first ASCT increased over time, while the use of tandem ASCT declined. The shift in treatment practices coincided with higher rates of complete response (CR) post-induction therapy. Significantly improved overall survival and event-free survival over time were observed across all age groups, especially in older patients, but not in patients under 40. Tandem ASCT showed benefits for patients who did not achieve CR after initial ASCT. However, patients with ISS III and renal impairment had poorer outcomes with tandem ASCT. In conclusion, while ASCT remains an important anti-myeloma tool, careful patient selection for tandem ASCT is essential, particularly avoiding its use in patients with ISS III and renal impairment, older age, and those already achieving CR after initial ASCT.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: All methods used were performed in accordance with the relevant guidelines and regulations. Data collection and analysis was approved by ethics committees of participating DRST centers and the study was performed according to the declaration of Helsinki. Informed consent was obtained from all participants.
Figures
References
-
- Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet Lond Engl. 2019;394:29–38. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

