Intratumoral delivery of lipid nanoparticle-formulated mRNA encoding IL-21, IL-7, and 4-1BBL induces systemic anti-tumor immunity
- PMID: 39639025
- PMCID: PMC11621563
- DOI: 10.1038/s41467-024-54877-9
Intratumoral delivery of lipid nanoparticle-formulated mRNA encoding IL-21, IL-7, and 4-1BBL induces systemic anti-tumor immunity
Abstract
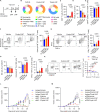
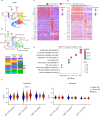
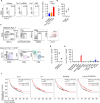
Local delivery of mRNA-based immunotherapy offers a promising avenue as it enables the production of specific immunomodulatory proteins that can stimulate the immune system to recognize and eliminate cancer cells while limiting systemic exposure and toxicities. Here, we develop and employ lipid-based nanoparticles (LNPs) to intratumorally deliver an mRNA mixture encoding the cytokines interleukin (IL)-21 and IL-7 and the immunostimulatory molecule 4-1BB ligand (Triplet LNP). IL-21 synergy with IL-7 and 4-1BBL leads to a profound increase in the frequency of tumor-infiltrating CD8+ T cells and their capacity to produce granzyme B and IFN-γ, leading to tumor eradication and the development of long-term immunological memory. Mechanistically, the efficacy of the Triplet LNP depends on tumor-draining lymph nodes to tumor CD8+ T-cell trafficking. Moreover, we highlight the therapeutic potential of the Triplet LNP in multiple tumor models in female mice and its superior therapeutic efficacy to immune checkpoint blockade. Ultimately, the expression of these immunomodulators is associated with better overall survival in patients with cancer.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: J.F., E.B., S.D.K., and F.L. are employees of etherna. J.F., E.B., S.D.K., and F.L. have applied for a patent related to the study (Compositions and methods for delivery of agents to immune cells; WO2023118411A1). S.D.K. and B.G.D.G. have applied for a patent related to the ionizable lipids used in this work (Ionizable lipids; WO2022136641A1). All other authors declare no potential conflicts of interest.
Figures





References
-
- Van Lint, S. et al. Intratumoral Delivery of TriMix mRNA Results in T-cell Activation by Cross-Presenting Dendritic Cells. Cancer Immunol. Res.4, 146–156 (2016). - PubMed
-
- Hotz, C. et al. Local delivery of mRNA-encoding cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl. Med.13, eabc7804 (2021). - PubMed
-
- Hewitt, S. L. et al. Intratumoral IL12 mRNA Therapy Promotes TH1 Transformation of the Tumor Microenvironment. Clin. Cancer Res.26, 6284–6298 (2020). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

