Toll-like receptor 3 signaling attenuated colitis-associated cancer development in mice
- PMID: 39639064
- PMCID: PMC11621332
- DOI: 10.1038/s41598-024-76954-1
Toll-like receptor 3 signaling attenuated colitis-associated cancer development in mice
Abstract
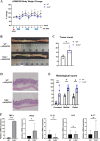
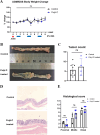
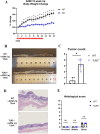
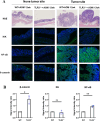
Inflammatory bowel disease is associated with a high risk of colitis-associated cancer (CAC). We evaluated the role of TLR3 in CAC using a murine model. Wild-type (WT) and TLR3-knockout (TLR3-/-) mice received azoxymethane (AOM) 12.5 mg/kg intraperitoneally on day zero, followed by three cycles of 2% dextran sulfate sodium (DSS) for five days and free water for two weeks. We evaluated clinical indices, such as weight change, colon length, histological severity of colitis, and tumor number. We performed immunofluorescence assays for phospho-IκB kinase and β-catenin in colon tissues. To elucidate the antitumorigenic mechanism of TLR3 signaling, we injected poly(I: C) or phosphate-buffered saline intraperitoneally into an AOM/DSS-induced tumorigenesis model in WT mice. We also evaluate the direct antitumor effect of TLR signaling in AOM-treated WT and TLR3-/- mice without DSS. TLR3 deficiency increased tumor burden and colitis severity in the colon tissue than in the WT mice. β-catenin immunoreactivity was higher in TLR3-/- mice, while phospho-IκB kinase expression was similar. TLR3 activation by poly(I: C) did not reduce tumor burden in WT mice, but long-term AOM administration without DSS significantly increased tumor burden in TLR3-/- mice. TLR3 signaling attenuates CAC development, suggesting it may be a target for preventing CAC in inflammatory bowel disease.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Olén, O. et al. Colorectal cancer in ulcerative colitis: a scandinavian population-based cohort study. Lancet. 395, 123–131 (2020). - PubMed
-
- Olén, O. et al. Colorectal cancer in Crohn’s disease: a scandinavian population-based cohort study. Lancet Gastroenterol. Hepatol. 5, 475–484 (2020). - PubMed
-
- Renz, B. W. et al. Clinical outcome of IBD-associated versus sporadic colorectal cancer: a matched-pair analysis. J. Gastrointest. Surg. 17, 981–990 (2013). - PubMed
-
- Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell. 124, 783–801 (2006). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

