ICU patient-on-a-chip emulating orchestration of mast cells and cerebral organoids in neuroinflammation
- PMID: 39639082
- PMCID: PMC11621364
- DOI: 10.1038/s42003-024-07313-z
ICU patient-on-a-chip emulating orchestration of mast cells and cerebral organoids in neuroinflammation
Abstract
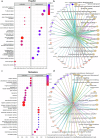
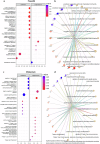
Propofol and midazolam are the current standard of care for prolonged sedation in Intensive Care Units (ICUs). However, the effects and mechanism of these sedatives in brain tissue are unclear. Herein, the development of an ICU patient-on-a-chip platform to elucidate those effects is reported. The humanized neural tissue compartment combines mast cells differentiated from human induced pluripotent stem cells (hiPSCs) with cerebral organoids in a three-dimensional (3D) matrix, which is covered with a membrane populated with human cerebral microvascular endothelial cells (hCMEC/D3) that separates the tissue chamber from the vascular lumen, where sedatives were infused for four days to evaluate neurotoxicity and cell-mediated immune responses. Subsequent to propofol administration, gene expressions of CD40 and TNF-α in mast cells, AIF1 in microglia and GFAP/S100B/OLIG2/MBP in macroglia were elevated, as well as NOS2, CD80, CD40, CD68, IL6 and TNF-α mediated proinflammation is noted in cerebral organoids, which resulted in higher expressions of GJB1, GABA-A and NMDAR1 in the tissue construct of the platform. Besides, midazolam administration stimulated expression of CD40 and CD203c+ reactivated mast cell proliferation and compromised BBB permeability and decreased TEER values with higher barrier disruption, whereas increased populations of CD11b+ microglia, higher expressions of GFAP/DLG4/GJB1 and GABA-A-/NMDAR1- identities, as well as glutamate related neurotoxicity and IL1B, IFNG, IFNA1, IL6 genes mediated proinflammation, resulting in increased apoptotic zones are observed in cerebral organoids. These results suggest that different sedatives cause variations in cell type activation that modulate different pathways related to neuroinflammation and neurotoxicity in the ICU patient-on-chip platform.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
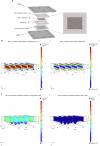

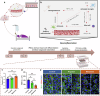
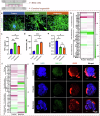
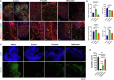
References
-
- Saglam-Metiner, P., Duran, E., Sabour-Takanlou, L., Biray-Avci, C. & Yesil-Celiktas, O. Differentiation of neurons, astrocytes, oligodendrocytes and microglia from human induced pluripotent stem cells to form neural tissue-on-chip: a neuroinflammation model to evaluate the therapeutic potential of extracellular vesicles derived from mesenchymal stem cells. Stem Cell Rev. Rep.20, 413–436 (2024). - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

