Bacterial products initiation of alpha-synuclein pathology: an in vitro study
- PMID: 39639092
- PMCID: PMC11621565
- DOI: 10.1038/s41598-024-81020-x
Bacterial products initiation of alpha-synuclein pathology: an in vitro study
Abstract
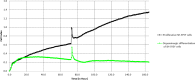
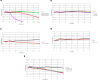
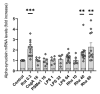
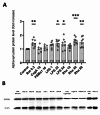
Parkinson's Disease (PD) is a prevalent and escalating neurodegenerative disorder with significant societal implications. Despite being considered a proteinopathy, in which the aggregation of α-synuclein is the main pathological change, the intricacies of PD initiation remain elusive. Recent evidence suggests a potential link between gut microbiota and PD initiation, emphasizing the need to explore the effects of microbiota-derived molecules on neuronal cells. In this study, we exposed dopaminergic-differentiated SH-SY5Y cells to microbial molecules such as lipopolysaccharide (LPS), rhamnolipid, curli CsgA and phenol soluble modulin α-1 (PSMα1). We assessed cellular viability, cytotoxicity, growth curves and α-synuclein levels by performing MTS, LDH, real-time impedance readings, qRT-PCR and Western Blot assays respectively. Statistical analysis revealed that rhamnolipid exhibited concentration-dependent effects, reducing viability and inducing cytotoxicity at higher concentrations, increasing α-synuclein mRNA and protein levels with negative effects on cell morphology and adhesion. Furthermore, LPS exposure also increased α-synuclein levels. Curli CsgA and PSMα-1 showed minimal or no changes. Our findings suggest that microbiota-derived molecules, particularly rhamnolipid and LPS, impact dopaminergic neurons by increasing α-synuclein levels. This study highlights the potential involvement of gut microbiota in initiating the upregulation of α-synuclein that may further initiate PD, indicating the complex interplay between microbiota and neuronal cells.
Keywords: Alpha-synuclein overexpression; Curli CsgA; Lipopolysaccharide; Microbiota; Microbiota-derived molecules; Parkinson’s disease; Phenol soluble modulin α-1; Rhamnolipid.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- 31PFE/30.12.2021/Ministry of Research, Innovation, and Digitalization in Romania
- 31PFE/30.12.2021/Ministry of Research, Innovation, and Digitalization in Romania
- 31PFE/30.12.2021/Ministry of Research, Innovation, and Digitalization in Romania
- 31PFE/30.12.2021/Ministry of Research, Innovation, and Digitalization in Romania
- 31PFE/30.12.2021/Ministry of Research, Innovation, and Digitalization in Romania
- 31PFE/30.12.2021/Ministry of Research, Innovation, and Digitalization in Romania
- 31PFE/30.12.2021/Ministry of Research, Innovation, and Digitalization in Romania
- 31PFE/30.12.2021/Ministry of Research, Innovation, and Digitalization in Romania
- 31PFE/30.12.2021/Ministry of Research, Innovation, and Digitalization in Romania
LinkOut - more resources
Full Text Sources

