New insights into the structural role of EMILINs within the human skin microenvironment
- PMID: 39639116
- PMCID: PMC11621341
- DOI: 10.1038/s41598-024-81509-5
New insights into the structural role of EMILINs within the human skin microenvironment
Abstract
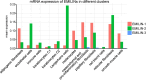
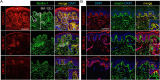
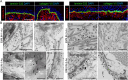
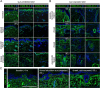
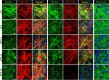
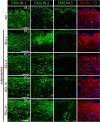
Supramolecular extracellular matrix (ECM) networks play an essential role in skin architecture and function. Elastin microfibril interface-located proteins (EMILINs) comprise a family of three extracellular glycoproteins that serve as essential structural components of the elastin/fibrillin microfibril network, and exert crucial functions in cellular signaling. Little is known about the structural nature of EMILIN networks in skin. We therefore investigated the spatiotemporal localization of EMILIN-1, -2, -3 in human skin induced by aging, UV-exposure, fibrosis, and connective tissue disorder. Confocal immunofluorescence and immunogold electron microscopy analysis identified all EMILINs as components of elastic fibers and elastin-free oxytalan fibers inserted into the basement membrane (BM). Further, our ultrastructural analysis demonstrates cellular contacts of dermally localized EMILIN-1 positive fibers across the BM with the surface of basal keratinocytes. Analysis of skin biopsies and fibroblast cultures from fibrillin-1 deficient Marfan patients revealed that EMILINs require intact fibrillin-1 as deposition scaffold. In patients with scleroderma and the bleomycin-induced murine fibrosis model EMILIN-2 was upregulated. EMILIN-3 localizes to the tips of candelabra-like oxytalan fibers, and to specialized BMs engulfing hair follicles and sebaceous glands. Our data identify EMILINs as important markers to monitor rearrangements of the dermal ECM architecture induced by aging and pathological conditions.
Keywords: Basement membrane; Connective tissue disease; Dermal matrix; Elastic fibers; Extracellular matrix proteins; Human skin; Oxytalan fibers; Skin fibrosis; UV exposure.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Spessotto, P. et al. beta 1 Integrin-dependent cell adhesion to EMILIN-1 is mediated by the gC1q domain. J. Biol. Chem.278, 6160–6167. 10.1074/jbc.M208322200 (2003). - PubMed
-
- Spessotto, P. et al. EMILIN1 represents a major stromal element determining human trophoblast invasion of the uterine wall. J. Cell Sci.119, 4574–4584. 10.1242/jcs.03232 (2006). - PubMed
-
- Schiavinato, A. et al. EMILIN-3, peculiar member of elastin microfibril interface-located protein (EMILIN) family, has distinct expression pattern, forms oligomeric assemblies, and serves as transforming growth factor beta (TGF-beta) antagonist. J. Biol. Chem.287, 11498–11515. 10.1074/jbc.M111.303578 (2012). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

