Clinical research framework proposal for ketogenic metabolic therapy in glioblastoma
- PMID: 39639257
- PMCID: PMC11622503
- DOI: 10.1186/s12916-024-03775-4
Clinical research framework proposal for ketogenic metabolic therapy in glioblastoma
Abstract
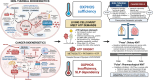
Glioblastoma (GBM) is the most aggressive primary brain tumor in adults, with a universally lethal prognosis despite maximal standard therapies. Here, we present a consensus treatment protocol based on the metabolic requirements of GBM cells for the two major fermentable fuels: glucose and glutamine. Glucose is a source of carbon and ATP synthesis for tumor growth through glycolysis, while glutamine provides nitrogen, carbon, and ATP synthesis through glutaminolysis. As no tumor can grow without anabolic substrates or energy, the simultaneous targeting of glycolysis and glutaminolysis is expected to reduce the proliferation of most if not all GBM cells. Ketogenic metabolic therapy (KMT) leverages diet-drug combinations that inhibit glycolysis, glutaminolysis, and growth signaling while shifting energy metabolism to therapeutic ketosis. The glucose-ketone index (GKI) is a standardized biomarker for assessing biological compliance, ideally via real-time monitoring. KMT aims to increase substrate competition and normalize the tumor microenvironment through GKI-adjusted ketogenic diets, calorie restriction, and fasting, while also targeting glycolytic and glutaminolytic flux using specific metabolic inhibitors. Non-fermentable fuels, such as ketone bodies, fatty acids, or lactate, are comparatively less efficient in supporting the long-term bioenergetic and biosynthetic demands of cancer cell proliferation. The proposed strategy may be implemented as a synergistic metabolic priming baseline in GBM as well as other tumors driven by glycolysis and glutaminolysis, regardless of their residual mitochondrial function. Suggested best practices are provided to guide future KMT research in metabolic oncology, offering a shared, evidence-driven framework for observational and interventional studies.
Keywords: Cancer; Glioblastoma; Glutaminolysis; Metabolism; Precision medicine; Research design; Warburg Effect.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: A.P. is an owner of Poff Medical Consulting and Communications, LLC, which performs consulting and public speaking services related to ketogenic metabolic therapy. A.P. is a scientific advisor to Pruvit Ventures, LLC, which sells exogenous ketone products. A.P. is an owner of Metabolic Health Initiative, LLC which is a medical education company in the field of metabolic health and metabolism-based therapies. A.P. is an inventor on and receives royalties from the following patent: “Targeting Cancer with Metabolic Therapy and Hyperbaric Oxygen” (Patent Number: 9801903). D.P.D. is an inventor of patents on the use of exogenous ketones, advisor for Levels Health, and co-owner of Ketone Technologies LLC, which does consulting and public speaking events. C.E.C. receives royalties from books, consulting, and lectures on nutrition and exercise, and serves on the scientific advisory board of Simply Good Foods/Atkins. M.K. is employed by Dietary Therapies LLC. The other authors declare no competing interests.
Figures




References
-
- Fatehi M, Hunt C, Ma R, Toyota BD. Persistent Disparities in Survival for Patients with Glioblastoma. World Neurosurg. 2018;120:e511–6. - PubMed
-
- Rocha Pinheiro SL, Lemos FFB, Marques HS, Silva Luz M, de Oliveira Silva LG. Faria Souza Mendes Dos Santos C, da Costa Evangelista K, Calmon MS, Sande Loureiro M, Freire de Melo F: Immunotherapy in glioblastoma treatment: Current state and future prospects. World J Clin Oncol. 2023;14(4):138–59. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

