Human liver organoids are susceptible to Plasmodium vivax infection
- PMID: 39639330
- PMCID: PMC11622667
- DOI: 10.1186/s12936-024-05202-8
Human liver organoids are susceptible to Plasmodium vivax infection
Erratum in
-
Correction: Human liver organoids are susceptible to Plasmodium vivax infection.Malar J. 2025 Oct 28;24(1):361. doi: 10.1186/s12936-025-05632-y. Malar J. 2025. PMID: 41152902 Free PMC article. No abstract available.
Abstract
Background: The eradication of Plasmodium vivax malaria is complicated due to the presence of hypnozoites, the hidden dormant form of the parasite that is present in the liver. Currently available drug regimens are effective at killing hypnozoites but cause side effects and are difficult to administer. Studies testing drugs for liver-stage malaria remain rare and mainly rely on the use of cancerous or immortalized hepatic cells and primary hepatocytes.
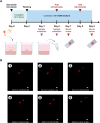
Methods: Organoids were used as platform to model liver-stage vivax malaria. Hepatic endoderm cells, endothelial progenitor cells and mesenchymal cells were generated from human induced pluripotent stem cells and self-assembled into liver organoids on top of Matrigel layer. Liver characteristic and maturity were examined through genes and proteins expression of liver markers, and liver functional tests before infected with Plasmodium vivax sporozoites. The infection was then verified by the detection of parasitophorous vacuole membrane proteins, Upregulated in Infectious Sporozoite 4 (UIS4), and blood-stage infection following co-culture with human reticulocytes.
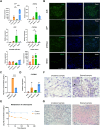
Results: Generated liver organoids showed upregulation of liver specific transcripts including hepatic nuclear factor 4A (HNF4A), alpha-fetoprotein (AFP), and albumin (ALB) which also confirmed by the protein expression. Furthermore, those organoids resembled mature hepatocytes in terms of albumin secretion, fat and glycogen storage and cytochrome activity. Following invasion of P. vivax sporozoites, PvUIS4 was detected and the hepatic merozoites could develop into ring-stage and early trophozoites in human reticulocytes. Moreover, differential expression patterns of genes involved in lipid and cholesterol synthesis were also detected.
Conclusions: Stem cell-derived liver organoids resemble mature liver cells in terms of liver functions and are susceptible to infection with P. vivax sporozoites, paving the way for studies on the mechanism of hypnozoite formation and testing of possible hypnozoitocidal drugs.
Keywords: Plasmodium vivax; Disease model; Hepatocyte; Induced pluripotent stem cells; Liver organoid; Malaria.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and and consent to participate: To generate liver organoids in this study, a human iPSC line, MUSIi001-A, established in a previous study [26], was used. Human erythrocytes were obtained from the peripheral venous blood of the subjects after obtaining informed consent. Protocols related to iPSCs, blood collection and cell preparation were approved by the Human Research Protection Unit, Faculty of Medicine Siriraj Hospital, Mahidol University (COA no. Si 953/2023, 924/2566(IRB3), and 400/2567(Exempt)). P. vivax sporozoites were used in accordance with biosafety guidelines, and the corresponding protocols were approved by the Faculty of Medicine Siriraj Hospital, Mahidol University (approval no. SI 2024–003). Consent for publications: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

