A fully human IgG1 antibody targeting connexin 32 extracellular domain blocks CMTX1 hemichannel dysfunction in an in vitro model
- PMID: 39639332
- PMCID: PMC11619691
- DOI: 10.1186/s12964-024-01969-0
A fully human IgG1 antibody targeting connexin 32 extracellular domain blocks CMTX1 hemichannel dysfunction in an in vitro model
Erratum in
-
Correction: A fully human IgG1 antibody targeting connexin 32 extracellular domain blocks CMTX1 hemichannel dysfunction in an in vitro model.Cell Commun Signal. 2024 Dec 12;22(1):591. doi: 10.1186/s12964-024-01988-x. Cell Commun Signal. 2024. PMID: 39668347 Free PMC article. No abstract available.
Abstract
Connexins (Cxs) are fundamental in cell-cell communication, functioning as gap junction channels (GJCs) that facilitate solute exchange between adjacent cells and as hemichannels (HCs) that mediate solute exchange between the cytoplasm and the extracellular environment. Mutations in the GJB1 gene, which encodes Cx32, lead to X-linked Charcot-Marie-Tooth type 1 (CMTX1), a rare hereditary demyelinating disorder of the peripheral nervous system (PNS) without an effective cure or treatment. In Schwann cells, Cx32 HCs are thought to play a role in myelination by enhancing intracellular and intercellular Ca2+ signaling, which is crucial for proper PNS myelination. Single-point mutations (p.S85C, p.D178Y, p.F235C) generate pathological Cx32 HCs characterized by increased permeability ("leaky") or excessive activity ("hyperactive").We investigated the effects of abEC1.1-hIgG1, a fully human immunoglobulin G1 (hIgG1) monoclonal antibody, on wild-type (WT) and mutant Cx32D178Y HCs. Using HeLa DH cells conditionally co-expressing Cx and a genetically encoded Ca2+ biosensor (GCaMP6s), we demonstrated that mutant HCs facilitated 58% greater Ca2+ uptake in response to elevated extracellular Ca2+ concentrations ([Ca2+]ex) compared to WT HCs. abEC1.1-hIgG1 dose-dependently inhibited Ca2+ uptake, achieving a 50% inhibitory concentration (EC50) of ~ 10 nM for WT HCs and ~ 80 nM for mutant HCs. Additionally, the antibody suppressed DAPI uptake and ATP release. An atomistic computational model revealed that serine 56 (S56) of the antibody interacts with aspartate 178 (D178) of WT Cx32 HCs, contributing to binding affinity. Despite the p.D178Y mutation weakening this interaction, the antibody maintained binding to the mutant HC epitope at sub-micromolar concentrations.In conclusion, our study shows that abEC1.1-hIgG1 effectively inhibits both WT and mutant Cx32 HCs, highlighting its potential as a therapeutic approach for CMTX1. These findings expand the antibody's applicability for treating diseases associated with Cx HCs and inform the rational design of next-generation antibodies with enhanced affinity and efficacy against mutant HCs.
Keywords: ATP release; Ca2+ uptake; Charcot–Marie–Tooth diseases; Connexons; Cx32; Dye uptake; Molecular dynamics; Monoclonal antibodies.
Plain language summary
Connexins (Cxs) are proteins essential for communication between cells, forming gap junction channels (GJCs) that connect neighboring cells and hemichannels (HCs) that link the inside of cells to their surroundings. Mutations in the GJB1 gene, which makes Cx32, cause X-linked Charcot-Marie-Tooth type 1 (CMTX1), a rare nerve disorder that currently has no cure. Our research studied the effects of a specially designed antibody, abEC1.1-hIgG1, on both normal and mutated Cx32 HCs using a well-established cell model for studying these channels. We discovered that the antibody significantly reduced the movement of calcium ions (Ca2+), which are vital for nerve health, in both normal and mutated channels. Further analysis showed that the antibody’s success might be due to how it binds to certain areas of the channels. Our findings suggest that abEC1.1-hIgG1 could be developed into a treatment for CMTX1 and similar conditions. Moreover, this research could help in creating even more effective antibodies for targeting faulty hemichannels.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: This study does not contain any individual participant data. No consent to publish is required. Competing interests: G. Yang, F. Zonta and F. Mammano report a patent family: “WO2017128880 – Fully human antibody specifically inhibiting connexin 26”, Inventors: Qu Z, Yang G, Mammano F, Zonta F, International application number: PCT/CN2016/109847, granted to ShanghaiTech University; and a patent family “WO2020237491 – Composition and Methods to treat Ectodermal Dysplasia 2, Clouston Type”, Inventors: Mammano F, Yang G, Zonta F, International Application No.: PCT/CN2019/088689, pending to ShanghaiTech University. The other authors declare no competing interests.
Figures


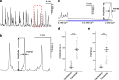

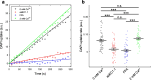




References
-
- Suter U, Snipes GJ. Biology and genetics of hereditary motor and sensory neuropathies. Annu Rev Neurosci. 1995;18:45–75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

