Inhibition of the chemokine receptors CXCR1 and CXCR2 synergizes with docetaxel for effective tumor control and remodeling of the immune microenvironment of HPV-negative head and neck cancer models
- PMID: 39639338
- PMCID: PMC11619435
- DOI: 10.1186/s13046-024-03240-3
Inhibition of the chemokine receptors CXCR1 and CXCR2 synergizes with docetaxel for effective tumor control and remodeling of the immune microenvironment of HPV-negative head and neck cancer models
Abstract
Background: Relapsed head and neck squamous cell carcinoma (HNSCC) unrelated to HPV infection carries a poor prognosis. Novel approaches are needed to improve the clinical outcome and prolong survival in this patient population which has poor long-term responses to immune checkpoint blockade. This study evaluated the chemokine receptors CXCR1 and CXCR2 as potential novel targets for the treatment of HPV-negative HNSCC.
Methods: Expression of IL-8, CXCR1, and CXCR2 was investigated in HNSCC tissues and human cell line models. Inhibition of CXCR1/2 with the clinical stage, small molecule inhibitor, SX-682, was evaluated in vitro and in vivo using human xenografts and murine models of HNSCC, both as a monotherapy and in combination with the taxane chemotherapy, docetaxel.
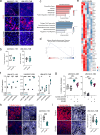
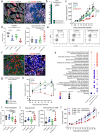
Results: High levels of IL-8, CXCR1, and CXCR2 expression were observed in HPV-negative compared to HPV-positive HNSCC tumors or cell lines. Treatment of HPV-negative HNSCC cell lines in vitro with SX-682 sensitized the tumor cells to the cytotoxic activity of docetaxel. In vivo, treatment of HNSCC xenograft models with the combination of SX-682 plus docetaxel led to strong anti-tumor control resulting in tumor cures. This phenomenon was associated with an increase of microRNA-200c and a decreased expression of its target, tubulin beta-3, a protein involved in resistance to microtubule-targeting chemotherapies. In vivo treatment of a murine syngeneic model of HNSCC with SX-682 plus docetaxel led to potent anti-tumor efficacy through a simultaneous decrease in suppressive CXCR2+ polymorphonuclear, myeloid-derived suppressor cells and an increase in cytotoxic CD8+ T cells in the combination therapy treated tumors compared to controls.
Conclusions: This study reports, for the first time, mechanistic findings through which the combination of CXCR1/2 inhibition and docetaxel chemotherapy exhibits synergy in models of HPV-negative HNSCC. These findings provide rationale for the use of this novel combination approach to treat HPV-negative HNSCC patients and for future combination studies of CXCR1/2 inhibition, docetaxel, and immune-based therapies.
Keywords: CXCR1; CXCR2; Docetaxel; HNSCC; IL-8; Immunotherapy.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures reported in this study were approved by the NCI Animal Care and Use Committee (ACUC) and in accordance with federal regulatory requirements and standards. All components of the intramural NIH ACU program are accredited by Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. Consent for publication: Not applicable. Competing interests: The authors from Syntrix Pharmaceuticals are employees or officers of the company. CP discloses spouse’s employment and holdings in MacroGenics, Inc. All other authors from the National Cancer Institute, NIH, do not have any competing interests to disclose. The NCI, NIH has an ongoing Cooperative Research and Development Agreement (CRADA) with Syntrix Pharmaceuticals.
Figures



References
-
- Bhatia A, Burtness B. Treating head and neck cancer in the age of immunotherapy: a 2023 update. Drugs. 2023;2023:217–48. - PubMed
-
- Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

