A Versatile "Synthesis Tag" (SynTag) for the Chemical Synthesis of Aggregating Peptides and Proteins
- PMID: 39639492
- PMCID: PMC11664589
- DOI: 10.1021/jacs.4c14247
A Versatile "Synthesis Tag" (SynTag) for the Chemical Synthesis of Aggregating Peptides and Proteins
Abstract
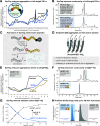
Solid-phase peptide synthesis (SPPS) and native chemical ligation (NCL) are powerful methods for obtaining peptides and proteins that are otherwise inaccessible. Nonetheless, numerous sequences are difficult to prepare via SPPS, and cleaved peptides often have low aqueous solubility. To address these challenges, we developed a "Synthesis Tag" consisting of six arginines connected to the target sequence via a cleavable MeDbz linker. "SynTag" effectively improves batch- and flow-SPPS of "difficult sequences", enhances the solubility of the cleaved peptides, and provides direct access to native sequences by hydrolysis, or peptide thioesters for NCL. We demonstrate its utility in the first chemical synthesis of the MYC transactivation domain with a single NCL. We envisage SynTag to become a broadly applicable tool that enables the synthesis and study of previously unattainable peptides and proteins.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Merrifield R. B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85 (14), 2149–2154. 10.1021/ja00897a025. - DOI
-
- Hartrampf N.; Saebi A.; Poskus M.; Gates Z. P.; Callahan A. J.; Cowfer A. E.; Hanna S.; Antilla S.; Schissel C. K.; Quartararo A. J.; Ye X.; Mijalis A. J.; Simon M. D.; Loas A.; Liu S.; Jessen C.; Nielsen T. E.; Pentelute B. L. Synthesis of Proteins by Automated Flow Chemistry. Science 2020, 368 (6494), 980–987. 10.1126/science.abb2491. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

