Transdermal delivery of PeptiCRAd cancer vaccine using microneedle patches
- PMID: 39639878
- PMCID: PMC11617629
- DOI: 10.1016/j.bioactmat.2024.11.006
Transdermal delivery of PeptiCRAd cancer vaccine using microneedle patches
Abstract
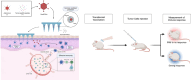
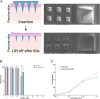
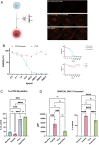
Microneedles (MNs) are a prospective system in cancer immunotherapy to overcome barriers regarding proper antigen delivery and presentation. This study aims at identifying the potential of MNs for the delivery of Peptide-coated Conditionally Replicating Adenoviruses (PeptiCRAd), whereby peptides enhance the immunogenic properties of adenoviruses presenting tumor associated antigens. The combination of PeptiCRAd with MNs containing polyvinylpyrrolidone and sucrose was tested for the preservation of structure, induction of immune response, and tumor eradication. The findings indicated that MN-delivered PeptiCRAd was effective in peptide presentation in vivo, leading to complete tumor rejection when mice were pre-vaccinated. A rise in the cDC1 population in the lymph nodes of the MN treated mice led to an increase in the effector memory T cells in the body. Thus, the results of this study demonstrate that the combination of MN technology with PeptiCRAd may provide a safer, more tolerable, and efficient approach to cancer immunotherapy, potentially translatable to other therapeutic applications.
Keywords: Adenoviral vector; Cancer therapy; Microneedles; Vaccine.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Hélder A. Santos reports a relationship with Research Council Finland and UMCG that includes: funding grants. Carmine D'Amico reports a relationship with 10.13039/501100003125Finnish Cultural Foundation that includes: funding grants. Vincenzo Cerullo reports a relationship with 10.13039/501100000781European Research Council (ERC), 10.13039/501100004155Magnus Ehrnrooth Foundation, 10.13039/501100004012Jane and Aatos Erkko Foundation, Finnish Cancer Foundation that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Guo Q., Wang C., Zhang Q., Cheng K., Shan W., Wang X., Yang J., Wang Y., Ren L. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of HBc VLPs based cancer vaccine. Appl. Mater. Today. 2021;24 doi: 10.1016/j.apmt.2021.101110. - DOI
LinkOut - more resources
Full Text Sources

