The role of Sertoli cell-derived miR-143-3p in male fertility declines with age
- PMID: 39640010
- PMCID: PMC11617286
- DOI: 10.1016/j.omtn.2024.102369
The role of Sertoli cell-derived miR-143-3p in male fertility declines with age
Abstract
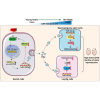
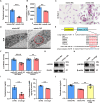
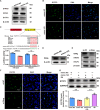
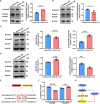
As delayed parenthood becomes more prevalent, understanding age-related testosterone decline and its impact on male fertility has gained importance. However, molecular mechanisms concerning testicular aging remain largely undiscovered. Our study highlights that miR-143-3p, present in aging Sertoli cells (SCs), is loaded into extracellular vesicles (EVs), affecting Leydig cells (LCs) and germ cells, thus disrupting testicular tissue homeostasis and spermatogenesis. Intriguingly, in SCs, transforming growth factor-β signaling promotes miR-143 precursors transcription, increasing mature miR-143-3p levels. This inhibits Smurf2, activating Smad2, and further enhancing miR-143-3p accumulation. EVs transporting miR-143-3p, originating from SCs, contribute to the age-related decline of testosterone and male fertility by targeting the luteinizing hormone receptor and retinoic acid receptor. Diminishing endogenous miR-143-3p in SCs postpones testis aging, preserving and prolonging male fertility. Thus, our study identified miR-143-3p as a key regulator of testicular function and fertility, revealing miR-143-3p as a potential therapeutic target for male abnormal sexual and reproductive function.
Keywords: EVs; MT: Non-coding RNAs; Sertoli cells; aging; extracellular vesicles; male fertility; miR-143-3p.
© 2024 Published by Elsevier Inc. on behalf of The American Society of Gene and Cell Therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Almeida S., Rato L., Sousa M., Alves M.G., Oliveira P.F. Fertility and Sperm Quality in the Aging Male. Curr. Pharmaceut. Des. 2017;23:4429–4437. - PubMed
-
- Kaufman J.M., Lapauw B., Mahmoud A., T'Sjoen G., Huhtaniemi I.T. Aging and the Male Reproductive System. Endocr. Rev. 2019;40:906–972. - PubMed
-
- Lunenfeld B. Endocrinology of the aging male. Minerva Ginecol. 2006;58:153–170. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

