First-in-class transactivator-free, doxycycline-inducible IL-18-engineered CAR-T cells for relapsed/refractory B cell lymphomas
- PMID: 39640015
- PMCID: PMC11617245
- DOI: 10.1016/j.omtn.2024.102308
First-in-class transactivator-free, doxycycline-inducible IL-18-engineered CAR-T cells for relapsed/refractory B cell lymphomas
Abstract
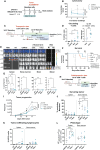
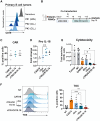
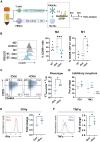
Although chimeric antigen receptor (CAR) T cell therapy has revolutionized type B cancer treatment, efficacy remains limited in various lymphomas and solid tumors. Reinforcing conventional CAR-T cells to release cytokines can improve their efficacy but also increase safety concerns. Several strategies have been developed to regulate their secretion using minimal promoters that are controlled by chimeric proteins harboring transactivators. However, these chimeric proteins can disrupt the normal physiology of T cells. Here, we present the first transactivator-free anti-CD19 CAR-T cells able to control IL-18 expression (iTRUCK19.18) under ultra-low doses of doxycycline and without altering cellular fitness. Interestingly, IL-18 secretion requires T cell activation in addition to doxycycline, allowing the external regulation of CAR-T cell potency. This effect was translated into an increased CAR-T cell antitumor activity against aggressive hematologic and solid tumor models. In a clinically relevant context, we generated patient-derived iTRUCK19.18 cells capable of eradicating primary B cells tumors in a doxycycline-dependent manner. Furthermore, IL-18-releasing CAR-T cells polarized pro-tumoral macrophages toward an antitumoral phenotype, suggesting potential for modulating the tumor microenvironment. In summary, we showed that our platform can generate exogenously controlled CAR-T cells with enhanced potency and in the absence of transactivators.
Keywords: CAR-T cells; IL-18; Lent-On-Plus; MT: Delivery Strategies; TRUCKs; animal models; doxycycline; lentiviral vectors; lymphoma; regulation.
© 2024 The Authors. Published by Elsevier Inc. on behalf of The American Society of Gene and Cell Therapy.
Conflict of interest statement
F.M. and P.M. are inventors of the patents entitled “Lent-on-plus system for conditional expression in human stem cells” (PCT/EP2017/078246) and “Insulator to improve gene transfer vectors” (PCT/EP2014/055027). F.M., M.T.-M., and J.A.M. are partners of LentiStem Biotech. P.J.-L., M.T.-M., and C.B.-J. are contractually associated with LentiStem Biotech, a spin-off company that holds the license of the above-mentioned patents.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

