A new nano approach to prevent tumor growth in the local treatment of glioblastoma: Temozolomide and rutin-loaded hybrid layered composite nanofiber
- PMID: 39640055
- PMCID: PMC11617954
- DOI: 10.1016/j.ajps.2024.100971
A new nano approach to prevent tumor growth in the local treatment of glioblastoma: Temozolomide and rutin-loaded hybrid layered composite nanofiber
Abstract
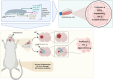
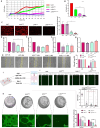
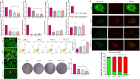
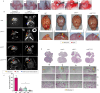
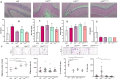
Total resection of glioblastoma (GB) tumors is nearly impossible, and systemic administration of temozolomide (TMZ) is often inadequate. This study presents a hybrid layered composite nanofiber mesh (LHN) designed for localized treatment in GB tumor bed. The LHN, consisting of polyvinyl alcohol and core-shell polylactic acid layers, was loaded with TMZ and rutin. In vitro analysis revealed that LHNTMZ and LHNrutin decelerated epithelial-mesenchymal transition and growth of stem-like cells, while the combination, LHNTMZ +rutin, significantly reduced sphere size compared to untreated and LHNTMZ-treated cells (P < 0.0001). In an orthotopic C6-induced GB rat model, LHNTMZ +rutin therapy demonstrated a more pronounced tumor-reducing effect than LHNTMZ alone. Tumor volume, assessed by magnetic resonance imaging, was significantly reduced in LHNTMZ +rutin-treated rats compared to untreated controls. Structural changes in tumor mitochondria, reduced membrane potential, and decreased PARP expression indicated the activation of apoptotic pathways in tumor cells, which was further confirmed by a reduction in PHH3, indicating decreased mitotic activity of tumor cells. Additionally, the local application of LHNs in the GB model mitigated aggressive tumor features without causing local tissue inflammation or adverse systemic effects. This was evidenced by a decrease in the angiogenesis marker CD31, the absence of inflammation or necrosis in H&E staining of the cerebellum, increased production of IFN-γ, decreased levels of interleukin-4 in splenic T cells, and lower serum AST levels. Our findings collectively indicate that LHNTMZ +rutin is a promising biocompatible model for the local treatment of GB.
Keywords: Glioblastoma; Hybrid layered composite nanofiber web; Local treatment; Rutin; Temozolomide.
© 2024 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Templeton A., Hofer S., Töpfer M., Sommacal A., Fretz C., Cerny T., et al. Extraneural spread of glioblastoma–report of two cases. Onkologie. 2008;31(4):192–194. - PubMed
-
- Castañeda C.A., Casavilca S., Orrego E., García-Corrochano P., Deza P., Heinike H., et al. Glioblastoma: análisis molecular y sus implicancias clínicas [Glioblastoma: molecular analysis and its clinical implications] Rev Peru Med Exp Salud Publica. 2015;32(2):316–325. - PubMed
-
- Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood-brain barrier: chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13:1099–1106. - PubMed
LinkOut - more resources
Full Text Sources

