Highly Reusable Electrochemical Immunosensor for Ultrasensitive Protein Detection
- PMID: 39640072
- PMCID: PMC11617009
- DOI: 10.1002/adsr.202400004
Highly Reusable Electrochemical Immunosensor for Ultrasensitive Protein Detection
Abstract
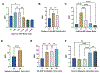
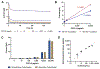
The detection and quantification of protein biomarkers in bodily fluids is important for many clinical applications, including disease diagnosis and health monitoring. Current techniques for ultrasensitive protein detection, such as enzyme-linked immunosorbent assay (ELISA) and electrochemical sensing, involve long incubation times (1.5-3 hr) and rely on single-use sensing electrodes which can be costly and generate excessive waste. This work demonstrates a reusable electrochemical immunosensor employing magnetic nanoparticles (MNPs) and dually labeled gold nanoparticles (AuNPs) for ultrasensitive measurements of protein biomarkers. As proof of concept, this platform was used to detect C-X-C motif chemokine ligand 9 (CXCL9), a biomarker associated with kidney transplant rejection, immune nephritis from checkpoint inhibitor therapy, and drug-associated acute interstitial nephritis, in human urine. The sensor successfully detected CXCL9 at concentrations as low as 27 pg/mL within ~1 hr. This immunosensor was also adapted onto a handheld smartphone-based diagnostic device and used for measurements of CXCL9, which exhibited a lower limit of detection of 65 pg/mL. Lastly, we demonstrate that the sensing electrodes can be reused for at least 100 measurements with a negligible loss in analytical performance, reducing the costs and waste associated with electrochemical sensing.
Keywords: CXCL9; diagnostic; electrochemical; gold nanoparticles; immunosensor; magnetic nanoparticles; reusable.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures
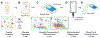
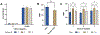
References
-
- Hayrapetyan H, Tran T, Tellez-Corrales E and Madiraju C, Enzyme-Linked Immunosorbent Assay: Types and Applications, Springer US, New York, NY: (2023) - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
