Acceptance of Organs from Deceased Donors With Resolved or Active SARS-CoV-2 Infection: A Survey From the Council of Europe
- PMID: 39640248
- PMCID: PMC11617184
- DOI: 10.3389/ti.2024.13705
Acceptance of Organs from Deceased Donors With Resolved or Active SARS-CoV-2 Infection: A Survey From the Council of Europe
Abstract
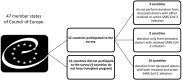
SARS-CoV-2 infection represents a new challenge for solid organ transplantation (SOT) with evolving recommendations. A cross-sectional survey was performed (February-June 2024) to describe practices among Member States of the Council of Europe (COE) on the use of organs from deceased donors with resolved or active SARS-CoV-2 infection. Overall, 32 out of 47 Member States with a transplant program participated in the study. Four (12.5%) countries did not use organs from deceased donors either with resolved or with active SARS-CoV-2 infection and 8 (25%) countries accepted organs only from deceased donors with resolved SARS-CoV-2 infection. Donor evaluation for SARS-CoV-2 included universal screening with standard PCR testing on respiratory specimens generally (61.4%) performed within 24 h prior to organ recovery. Further microbiological, immunological and radiological investigations varied. Most waitlisted patients receiving organs from a deceased donor with active (94.5%) or resolved (61.5%) SARS-CoV-2 infection were preferred to have natural, vaccine-induced or hybrid SARS-CoV-2 immunity. Most countries did not require recipients to undergo specific anti-SARS-CoV-2 treatment as pre-exposure (0%), post-exposure prophylaxis (15.4%) or modification of immunosuppression regimen (24%). This study highlights similarities and heterogeneities in the management of SARS-CoV-2 positive donors between COE countries, and a potential to safely expand donors' pool.
Keywords: COVID-19; DDI; Sars-CoV-2; donor; donor derived infections; recipient.
Copyright © 2024 Peghin, Graziano, De Martino, Balsamo, Isola, López-Fraga, Cardillo, Feltrin, Domínguez-Gil González, Grossi and The COVIDonors COE Study Group.
Conflict of interest statement
MP has the following conflict of interest: speaker for Dia Sorin, Merck, Sharp & Dohme, Menarini, Pfizer, Thermofisher; PG has the following conflict of interest: Consulting fees from Merck, Sharp & Dohme, Gilead Sciences, Takeda, Shionogi, Allovir, Astra-Zeneca, Menarini; member of speakers bureau for Merck, Sharp & Dohme, Gilead Sciences, Takeda, Astra-Zeneca. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous