Immunoinformatic design of a multivalent vaccine against Brucella abortus and its evaluation in a murine model using a DNA prime-protein boost strategy
- PMID: 39640259
- PMCID: PMC11617539
- DOI: 10.3389/fimmu.2024.1456078
Immunoinformatic design of a multivalent vaccine against Brucella abortus and its evaluation in a murine model using a DNA prime-protein boost strategy
Abstract
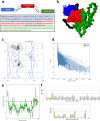
Introduction: The development of effective vaccines against Brucella abortus is critical due to its significant impact on human and animal health. The objective of this study was to design and evaluate in silico and in vivo a multivalent vaccine based on the immunogenic potential of three selected open reading frames (ORFs) of Brucella.
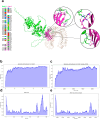
Methods: The designed construct, named S22, was analyzed in silico to evaluate its physicochemical properties, antigenicity, allergenicity and toxicity. This construct was modeled and subjected to molecular dynamics analysis. Additionally, the antigenicity and protection induced by this construct was evaluated through In vivo assays immunizing BALB/c mice with protein (S22), DNA (pVS22) and combining both vaccine formats using a prime boost immunization strategy.
Results: All bioinformatics analyses showed safe and high quality structural features, revealing favorable interactions between S22 and the TLR4/MD2 complex. Moreover, results from in vivo assays indicated that the S22 protein induced robust levels of IgG1 and IgG2a, suggesting a balanced Th1 and Th2 immune response. The DNA construct (pVS22) elicited primarily a Th1 response, whereas the use of a prime boost strategy, which combines both formats resulted in a balanced immune response with significant induction of lymphoproliferation and elevated.
Discussion: Although our assays did not demonstrate the induction of a substantial protective response against B. abortus, this construct was capable of inducing immunogenicity. This study highlights the utility of in silico design for predicting and optimizing candidate vaccines and underscores the potential of using strategies such as prime boost, which incorporate antigens of different biological nature to modulate the immune response, while balancing parameters such as stability of the antigens and the cost of production.
Keywords: immunogenicity; immunoinformatic; interferon gamma; molecular dynamics; multivalent vaccines; prime-boost strategy; protection.
Copyright © 2024 Molina, Osorio, Flores-Concha, Gómez, Alvarado, Ferrari and Oñate.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could potentially create a conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

