Neuronal substance P-driven MRGPRX2-dependent mast cell degranulation products differentially promote vascular permeability
- PMID: 39640264
- PMCID: PMC11617324
- DOI: 10.3389/fimmu.2024.1477072
Neuronal substance P-driven MRGPRX2-dependent mast cell degranulation products differentially promote vascular permeability
Abstract
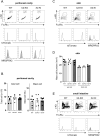
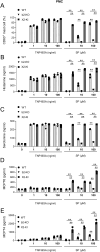
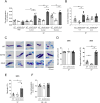
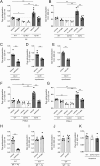
Mas-related G protein-coupled receptor b2 (Mrgprb2) binding to its cationic endogenous and exogenous ligands induces mast cell degranulation and promotes inflammation in mice. However, the physiological roles of its human homologue MRGPRX2 remain unclear. Here we aimed to elucidate the mechanisms by which MRGPRX2 regulates vascular permeability, and generated MRGPRX2 knock-in (MRGPRX2-KI) and Mrgprb2 knockout (Mrgprb2-KO) mice. Substance P (SP) and ciprofloxacin strongly degranulated MRGPRX2-KI peritoneal mast cells (PMCs) better than WT PMCs, whereas Dermatophagoides pteronyssinus (Der p) extract and phenol-soluble modulin α3 (PSMα3) did not degranulate PMCs. SP-stimulated MRGPRX2-KI PMCs released large amounts of histamine and mast cell protease 4 (MCPT4) chymase. Der p extract, PSMα3, and MCPT4, but not histamine, induced SP release from dorsal root ganglion (DRG) cells. However, this effect of Der p extract/PSMα3 was suppressed by a transient receptor potential vanilloid 1 (TRPV1) antagonist. SP-, ciprofloxacin-, Der p extract-, PSMα3-, and MCPT4-induced vascular permeability was highest in MRGPRX2-KI mice, which depended on SP. In addition, SP-, ciprofloxacin- and PSMα3-induced MRGPRX2-dependent vascular hyperpermeability was suppressed by antihistamine and chymase inhibitor. TRPV1 antagonist also inhibited PSMα3-induced MRGPRX2-dependent vascular hyperpermeability. Both Mrgprb2-KO and MRGPRX2-KI did not influence the histamine-induced murine vascular hyperpermeability. Overall, our results suggest that neuronal SP induces MRGPRX2-dependent mast cell degranulation, releasing histamine and chymase, which promote vascular hyperpermeability directly or indirectly via DRG cell activation. Importantly, the worsening cycle (MRGPRX2 → mast cell degranulation → chymase → DRG activation → SP → MRGPRX2) seems to play an important role in human MRGPRX2-depdendent inflammation.
Keywords: MRGPRX2; chymase; degranulation; histamine; mast cell; sensory neuron; substance P; vascular permeability.
Copyright © 2024 Nagamine, Kaitani, Izawa, Ando, Yoshikawa, Nakamura, Maehara, Yamamoto, Okamoto, Wang, Yamada, Maeda, Nakano, Shimizu, Ogawa, Okumura and Kitaura.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

