PTX3 is expressed in terminal lymphatics and shapes their organization and function
- PMID: 39640269
- PMCID: PMC11617523
- DOI: 10.3389/fimmu.2024.1426869
PTX3 is expressed in terminal lymphatics and shapes their organization and function
Abstract
Introduction: The lymphatic system is a multifaceted regulator of tissue homeostasis and an integral part of immune responses. Previous studies had shown that subsets of lymphatic endothelial cells (LEC) express PTX3, an essential component of humoral innate immunity and tissue homeostasis.
Methods: In the present study using whole-mount imaging and image-based morphometric quantifications, Ptx3-targeted mice and in vivo functional analysis, we investigated the involvement of PTX3 in shaping and function of the lymphatic vasculature.
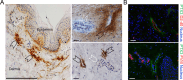
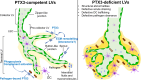
Results: We found that PTX3 is localized in the extracellular matrix (ECM) surrounding human and murine lymphatic vessels (LV). In murine tissues, PTX3 was localized in the ECM close to LV terminals and sprouting. Ptx3-deficient mice showed LV abnormalities in the colon submucosa and diaphragm, including a disorganized pattern and hyperplasia of initial LV capillaries associated with altered distribution of tight junction-associated molecules. Mice with LEC-restricted PTX3 gene inactivation showed morphological and organization abnormalities similar to those observed in Ptx3-deficient animals. Ptx3-deficient mice showed defective fluid drainage from footpads and defective dendritic cell (DC) trafficking.
Discussion: Thus, PTX3 is strategically localized in the ECM of specialized LV, playing an essential role in their structural organization and immunological function.
Keywords: extracellular matrix; innate immunity; lymphatic system; pattern recognition molecule; tissue homeostasis.
Copyright © 2024 Doni, Sironi, Del Prete, Pasqualini, Valentino, Cuccovillo, Parente, Calvi, Tosoni, Vago, Nebuloni, Garlanda, Vecchi, Bottazzi and Mantovani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

