Selective aqueous anion recognition in an anionic host
- PMID: 39640565
- PMCID: PMC11617965
- DOI: 10.1016/j.isci.2024.111348
Selective aqueous anion recognition in an anionic host
Abstract
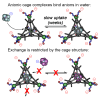
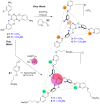
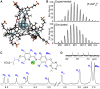
Water-soluble Fe4L4 4- cages can be synthesized in a multicomponent self-assembly process exploiting functionalized trigonal ligands, FeII salts, and water-soluble sulfonated formylpyridine components. The cages are soluble in purely aqueous solution and display an overall 4- charge, but are capable of binding suitably sized non-coordinating anions in the host cavity despite their anionic nature. Anions such as PF6 - or AsF6 - occupy the internal cavity, whereas anions that are too small (BF4 -) or too large (NTf2 -) are not encapsulated. The external anionic charge and sterically blocked ligand cores limit the exchange rate of bound anions, as no exchange is seen over a period of weeks with the anion-filled cages, and internalization of added PF6 - by an empty cage takes multiple weeks, despite the strong affinity of the cavity for PF6 - ions. In the future, this recognition mechanism could be used to control release of anions for environmental applications.
Keywords: Chemistry; Supramolecular chemistry.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

