The role of the tricellular junction protein ILDR2 in glomerulopathies: Expression patterns and functional insights
- PMID: 39640577
- PMCID: PMC11617378
- DOI: 10.1016/j.isci.2024.111329
The role of the tricellular junction protein ILDR2 in glomerulopathies: Expression patterns and functional insights
Abstract
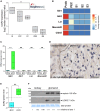
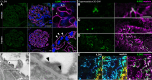
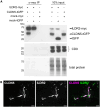
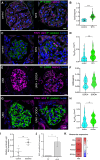
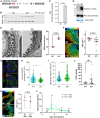
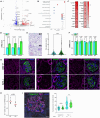
The tricellular tight junctions are crucial for the regulation of paracellular flux at tricellular junctions, where tricellulin (MARVELD2) and angulins (ILDR1, ILDR2, or LSR) are localized. The role of ILDR2 in podocytes, specialized epithelial cells in the kidney, is still unknown. We investigated the role of ILDR2 in glomeruli and its influence on blood filtration. Western blots, single-cell RNA sequencing (scRNA-seq), and superresolution microscopy showed a strong expression of ILDR2 in podocytes that colocalized with the podocyte-specific claudin CLDN5. Co-immunoprecipitation revealed that ILDR2 interacts with CLDN5. In glomerulopathies, induced by nephrotoxic serum and by desoxycorticosterone acetate (DOCA)-salt heminephrectomy, ILDR2 was strongly up-regulated. Furthermore, Ildr2 knockout mice exhibited glomerular hypertrophy and decreased podocyte density. However, they did not develop effacement of podocyte foot processes or proteinuria. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) proteomic analysis of isolated glomeruli showed an increase in matrix proteins, such as fibronectin and collagens. This suggests a protective role of ILDR2 in glomerulopathies.
Keywords: biological sciences; cellular physiology; natural sciences; physiology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Higashi T., Tokuda S., Kitajiri S., Masuda S., Nakamura H., Oda Y., Furuse M. Analysis of the 'angulin' proteins LSR, ILDR1 and ILDR2--tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J. Cell Sci. 2013;126:966–977. doi: 10.1242/jcs.116442. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

