The antibacterial activity of the copper for Staphylococcus aureus 124 and Pseudomonas aeruginosa 18 depends on its state: metalized, chelated and ionic
- PMID: 39640629
- PMCID: PMC11620121
- DOI: 10.1016/j.heliyon.2024.e39098
The antibacterial activity of the copper for Staphylococcus aureus 124 and Pseudomonas aeruginosa 18 depends on its state: metalized, chelated and ionic
Abstract
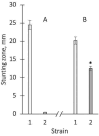
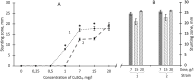
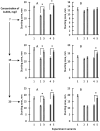
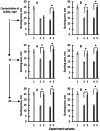
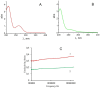
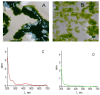
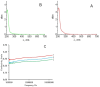
The hypothesis that the antibacterial effect of copper depends on its state was tested. It was studied the antibacterial effect of copper applied to the fabric, copper in chelated and free (ionic) forms on the growth intensity of Staphylococcus aureus 124 and Pseudomonas aeruginosa 18 in the in vitro system after a single or "primary" contact. Classical microbiology methods were used. Copper was applied to the fabric by magnetron and arc planar discharge systems, and the culture of microalgae Dunaliella viridis, resistant to the action of high concentrations of copper, was used to obtain copper in chelated form. It was shown that a thin layer of copper (3 μm) applied to the fabric showed pronounced antibacterial activity against Staphylococcus (85 % compared to the antibiotic meropenem) and less pronounced activity against Pseudomonas, which is resistant to meropenem. Copper in ionic form inhibited the growth of Staphylococcus aureus 124 as well as the antibiotic, and also effectively inhibited the growth of Pseudomonas aeruginosa 18 i.e., copper ions did not have species specificity like the antibiotic. Components of Dunaliella viridis microalgae cells had weakly expressed antibacterial effect to these types of bacteria, and supplementary addition of copper sulfate to the biomass of microalgae led to an increase of their antibacterial activity and this is more pronounced for microalgae culture in which the ratio « chelated/ionic » forms of copper is shifted to the ionic form. It was shown that the antibacterial activity of microalgae biomass after the first introduction into the tested bacterial cultures depends on the amount of free or "weakly bound" with cell components copper ions. It is suggested that the antibacterial effect of fabric with a thin layer of copper may be determined by two mechanisms: the action of copper ions and mechano-bactericidal effects, while chelated forms of copper may have a prolonged effect on bacterial cultures.
© 2024 The Authors.
Conflict of interest statement
The work was carried out within the framework of the project of the Ministry of Education and Science of Ukraine No. 0123U101860. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
LinkOut - more resources
Full Text Sources

