CoFe-MOF nanoarray as flexible microelectrode for electrochemical detection of catechol in water samples
- PMID: 39640810
- PMCID: PMC11620242
- DOI: 10.1016/j.heliyon.2024.e39241
CoFe-MOF nanoarray as flexible microelectrode for electrochemical detection of catechol in water samples
Abstract
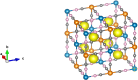
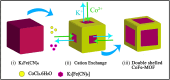
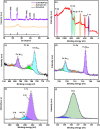
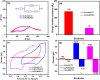
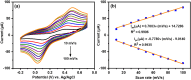
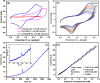
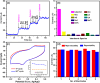
A simple, selective, and straightforward enzyme-free electrochemical sensor has been designed and developed using cobalt hexacyanoferrate metal-organic framework (CoFe-MOF) nanoarray. The prepared CoFe-MOF nanoarray has been successfully grown over a carbon cloth (CC) to form CoFe-MOF/CC as a flexible microelectrode for the detection of catechol. The surface of the activated CC was covered uniformly with CoFe-MOF in the form of nanoarray and exhibited double-shelled cubic morphology. The CoFe-MOF/CC nanoarray microelectrode showed a pair of well-defined redox peaks corresponding to the [Fe(CN)6]4-/3- redox signal. Interestingly, the fabricated nanoarray microelectrode has displayed superior peak current at lower onset potential with high electrochemical response compared to unmodified potassium hexacyanoferrate (K3 [Fe(CN)6]) over CC microelectrode and bare activated CC. Further, the developed CoFe-MOF/CC nanoarray microelectrode for the oxidation of catechol was examined with consecutive injections of catechol. A fast and noticeable improvement in oxidation peak current was observed, thus representing the excellent electrocatalytic oxidation of catechol at the modified nanoarray microelectrode. Besides, CoFe-MOF/CC microelectrode exhibits an excellent linear response over a concentration range from 0.005 to 2.8 mM with low detection limit (LOD) and high sensitivity of 0.002 mM (S/N = 3) and 205.99 μA/mM, respectively. Moreover, the prepared nonenzymatic sensor showed outstanding stability, acceptable reproducibility, and repeatability, along with good interference ability. Catechol in spiked water samples was successfully quantified.
Keywords: Catechol; Cyclic voltammetry; Electrochemical sensor; Nanoarray microelectrode; Nonenzymatic sensor; Redox mediator; Water samples.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Zhang M., Ye J., Fang P., Zhang Z., Wang C., Wu G. Facile electrochemical preparation of NaOH nanorods on glassy carbon electrode for ultrasensitive and simultaneous sensing of hydroquinone, catechol and resorcinol. Electrochim. Acta. 2019;317:618–627. doi: 10.1016/j.electacta.2019.06.006. - DOI
-
- Li N., Huang J.R., Zhang H.Y., Cui M., Sun B., Zhang C., Zhao H.Y. Decoration of covalent polyoxometalate-organic frameworks with Pt nanoparticles and multiwalled carbon nanotubes for simultaneous electrochemical detection of hydroquinone and catechol. ACS Appl. Nano Mater. 2024;7:1960–1969. doi: 10.1021/acsanm.3c05232. - DOI
-
- Kaneva M., Levshakova A., Tumkin I., Fatkullin M., Gurevich E., Manshina A., Rodriguez R.D., Khairullina E. Simultaneous electrochemical detection of hydroquinone and catechol using flexible laser-induced metal-polymer composite electrodes. Microchem. J. 2024;30 doi: 10.1016/j.microc.2024.111106. - DOI
LinkOut - more resources
Full Text Sources

