Longitudinal multi-trajectory phenotypes of severe eosinophilic asthma on type 2 biologics treatment
- PMID: 39640896
- PMCID: PMC11617764
- DOI: 10.1016/j.waojou.2024.101000
Longitudinal multi-trajectory phenotypes of severe eosinophilic asthma on type 2 biologics treatment
Abstract
Background: Limited understanding exists regarding the progression trajectory of severe eosinophilic asthma (SEA) patients on type 2 biologics therapies.
Objective: We aim to explore distinct longitudinal phenotypes of these patients based on crucial asthma biomarkers.
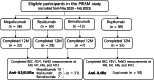
Methods: We enrolled 101 adult patients with SEA. Of these, 51 were treated with anti-IL5/IL5Rα or anti-IL5/IL5RαR antibody, and 50 with anti-IL-4Rα antibody. Multi-trajectory analysis, an extension of univariate group-based trajectory modeling, was used to categorize patients based on their trajectories of forced expiratory volume in 1 s (FEV1), blood eosinophil counts (BEC), and fractional exhaled nitric oxide (FeNO) levels at baseline, and after 1, 6, and 12 months of treatment. Associations between trajectory-based clusters and clinical parameters were examined.
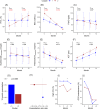
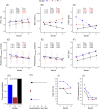
Results: Among anti-IL5/IL5Rα antibody-treated patients, 2 clusters were identified. The cluster characterized by higher baseline BEC and lower FEV1 showed a better response, with improvements in FEV1 and reductions in BEC over time. Among anti-IL-4Rα antibody-treated, 3 clusters were identified. Clusters with moderate BEC and FeNO at baseline demonstrated better improvements in FEV1 and reductions in FeNO, despite increased BEC during follow-up. Conversely, individuals with extremely low FeNO and high BEC at baseline were more likely to experience poorer progression, demonstrating an increase in FeNO and a reduction in FEV1.
Conclusion: To optimally monitor treatment response in SEA patients on type 2 biologics, integrating longitudinal biomarker features is essential.
Keywords: Multi-trajectory analysis; Severe eosinophilic asthma; Type 2 biologics.
© 2024 The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Chung K.F., Wenzel S.E., Brozek J.L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. - PubMed
-
- Wang E., Wechsler M.E., Tran T.N., et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157(4):790–804. - PubMed
-
- Calzetta L., Matera M.G., Rogliani P. Monoclonal antibodies in severe asthma: is it worth it? Expet Opin Drug Metabol Toxicol. 2019;15(6):517–520. - PubMed
-
- Coverstone A.M., Seibold M.A., Peters M.C. Diagnosis and management of T2-high asthma. J Allergy Clin Immunol Pract. 2020;8(2):442–450. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

