SSB expression is associated with metabolic parameters of 18F-FDG PET/CT in lung adenocarcinoma and can improve diagnostic efficiency
- PMID: 39641036
- PMCID: PMC11617761
- DOI: 10.1016/j.heliyon.2024.e38702
SSB expression is associated with metabolic parameters of 18F-FDG PET/CT in lung adenocarcinoma and can improve diagnostic efficiency
Abstract
Purpose: The study evaluates the expression and functional significance of the Small RNA Binding Exonuclease Protection Factor La (SSB) gene in lung adenocarcinoma (LUAD). By utilizing 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) machines, we correlated SSB gene expression with PET/CT parameters, as well as its value in LUAD diagnosis.
Methods: Fifty-five patients with LUAD underwent 18F-FDG PET/CT imaging prior to pulmonary surgery. Metabolic parameters such as maximum standardized uptake values (SUVmax) were quantitatively calculated from the 18F-FDG PET/CT imaging data. The diagnostic value was compared with that of thyroid transcription factor 1 (TTF1, the current standard-of-care). Publicly procurable datasets from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) were used to establish SSB gene expression patterns across diverse cancer types and specifically in LUAD, along with its associations with glycolysis and N6-methyladenosine (m6A) modification.
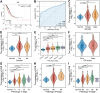
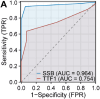
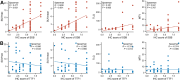
Results: SSB was highly expressed in LUAD compared to adjacent non-cancerous tissues. SSB additionally demonstrated superior diagnostic utility for LUAD compared to TTF1. The correlation between SSB and SUVmax as well as average standardized uptake values (SUVmean) was positive (P < 0.001), while TTF1 displayed a negative correlation with metabolic tumor volume (MTV) and total lesion glycolysis (TLG) (P < 0.05).
Conclusion: In LUAD, SSB expression correlated with high metabolic activity (SUV) on 18F-FDG PET/CT imaging. SSB is not only an important prognostic marker for lung cancer metastases, but may also represent a novel therapeutic target.
Keywords: 18F-FDG PET/CT; Glucose; LUAD; Metabolic parameter; SSB.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Nucleophosmin 1 overexpression correlates with 18F-FDG PET/CT metabolic parameters and improves diagnostic accuracy in patients with lung adenocarcinoma.Eur J Nucl Med Mol Imaging. 2021 Mar;48(3):904-912. doi: 10.1007/s00259-020-05005-4. Epub 2020 Aug 27. Eur J Nucl Med Mol Imaging. 2021. PMID: 32856112
-
Comparison of the diagnostic accuracy between 18F-FAPI-04 PET/CT and 18F-FDG PET/CT in the clinical stage IA of lung adenocarcinoma.J Thorac Dis. 2025 Feb 28;17(2):661-675. doi: 10.21037/jtd-24-1658. Epub 2025 Feb 27. J Thorac Dis. 2025. PMID: 40083505 Free PMC article.
-
Prognostic significance of volume-based 18F-FDG PET/CT parameters and correlation with PD-L1 expression in patients with surgically resected lung adenocarcinoma.Medicine (Baltimore). 2021 Sep 3;100(35):e27100. doi: 10.1097/MD.0000000000027100. Medicine (Baltimore). 2021. PMID: 34477147 Free PMC article.
-
SFXN1 as a potential diagnostic and prognostic biomarker of LUAD is associated with 18F-FDG metabolic parameters.Lung Cancer. 2024 Feb;188:107449. doi: 10.1016/j.lungcan.2023.107449. Epub 2023 Dec 27. Lung Cancer. 2024. PMID: 38184958
-
The role of baseline 18F-FDG PET/CT for survival prognosis in NSCLC patients undergoing immunotherapy: a systematic review and meta-analysis.Ther Adv Med Oncol. 2024 Nov 4;16:17588359241293364. doi: 10.1177/17588359241293364. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39502406 Free PMC article. Review.
References
-
- Lortet-Tieulent J., Soerjomataram I., Ferlay J., Rutherford M., Weiderpass E., Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84:13–22. doi: 10.1016/j.lungcan.2014.01.009. - DOI - PubMed
-
- Zhang Y., Vaccarella S., Morgan E., Li M., Etxeberria J., Chokunonga E., Manraj S.S., Kamate B., Omonisi A., Bray F. Global variations in lung cancer incidence by histological subtype in 2020: a population-based study. Lancet Oncol. 2023;24:1206–1218. doi: 10.1016/S1470-2045(23)00444-8. - DOI - PubMed
-
- Li Y., Sheng H., Ma F., Wu Q., Huang J., Chen Q., Sheng L., Zhu X., Zhu X., Xu M. RNA m6A reader YTHDF2 facilitates lung adenocarcinoma cell proliferation and metastasis by targeting the AXIN1/Wnt/β-catenin signaling. Cell Death Dis. 2021;12:479. doi: 10.1038/s41419-021-03763-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

