The pivotal role of the Hes1/Piezo1 pathway in the pathophysiology of glucocorticoid-induced osteoporosis
- PMID: 39641269
- PMCID: PMC11623955
- DOI: 10.1172/jci.insight.179963
The pivotal role of the Hes1/Piezo1 pathway in the pathophysiology of glucocorticoid-induced osteoporosis
Abstract
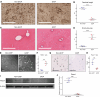
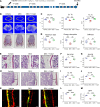
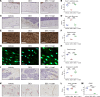
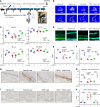
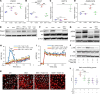
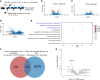
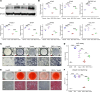
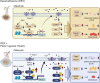
Glucocorticoid-induced osteoporosis (GIOP) lacks fully effective treatments. This study investigated the role of Piezo1, a mechanosensitive ion channel component 1, in GIOP. We found reduced Piezo1 expression in cortical bone osteocytes from patients with GIOP and a GIOP mouse model. Yoda1, a Piezo1 agonist, enhanced the mechanical stress response and bone mass and strength, which were diminished by dexamethasone (DEX) administration in GIOP mice. RNA-seq revealed that Yoda1 elevated Piezo1 expression by activating the key transcription factor Hes1, followed by enhanced CaM kinase II and Akt phosphorylation in osteocytes. This improved the lacuno-canalicular network and reduced sclerostin production and the receptor activator of NF-κB/osteoprotegerin ratio, which were mitigated by DEX. Comparative analysis of mouse models and human GIOP cortical bone revealed downregulation of mechanostimulated osteogenic factors, such as osteocrin, and cartilage differentiation markers in osteoprogenitor cells. In human periosteum-derived cells, DEX suppressed differentiation into osteoblasts, but Yoda1 rescued this effect. Our findings suggest that reduced Piezo1 expression and activity in osteocytes and periosteal cells contribute to GIOP, and Yoda1 may offer a novel therapeutic approach by restoring mechanosensitivity.
Keywords: Bone biology; Bone disease; Osteoporosis; Therapeutics.
Conflict of interest statement
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

