Dopamine-conjugated extracellular vesicles induce autophagy in Parkinson's disease
- PMID: 39641313
- PMCID: PMC11621972
- DOI: 10.1002/jev2.70018
Dopamine-conjugated extracellular vesicles induce autophagy in Parkinson's disease
Erratum in
-
Correction to "Dopamine-Conjugated Extracellular Vesicles Induce Autophagy in Parkinson's Disease".J Extracell Vesicles. 2025 May;14(5):e70082. doi: 10.1002/jev2.70082. J Extracell Vesicles. 2025. PMID: 40384180 Free PMC article. No abstract available.
Abstract
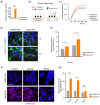
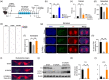
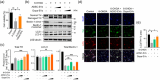
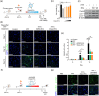
The application of extracellular vesicles (EVs) as vehicles for anti-Parkinson's agents represents a significant advance, yet their clinical translation is hampered by challenges in efficient brain delivery and complex blood-brain barrier (BBB) targeting strategies. In this study, we engineered dopamine onto the surface of adipose-derived stem cell EVs (Dopa-EVs) utilizing a facile, two-step cross-linking approach. This engineering enhanced neuronal uptake of the EVs in primary neurons and neuroblastoma cells, a process shown to be competitively inhibited by dopamine pretreatment and dopamine receptor antibodies. Notably, Dopa-EVs demonstrated increased brain accumulation in mouse Parkinson's disease (PD) models. Therapeutically, Dopa-EVs administration led to the rescue of dopaminergic neuronal loss and amelioration of behavioural deficits in both 6-hydroxydopamine (6-OHDA) and α-Syn PFF-induced PD models. Furthermore, we observed that Dopa-EVs stimulated autophagy evidenced by the upregulation of Beclin-1 and LC3-II. These findings collectively indicate that surface modification of EVs with dopamine presents a potent strategy for targeting dopaminergic neurons in the brain. The remarkable therapeutic potential of Dopa-EVs, demonstrated in PD models, positions them as a highly promising candidate for PD treatment, offering a significant advance over current therapeutic modalities.
Keywords: Parkinson's disease; autophagy; dopamine; exosome; extracellular vesicles.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors declare the following competing interests: D.‐G. Jo and J. H. Park are stockholders of ExoStemTech Inc. The other authors declare no competing financial interests. The graphical abstract was created with BioRender.com.
Figures



References
-
- Anraku, Y. , Kuwahara, H. , Fukusato, Y. , Mizoguchi, A. , Ishii, T. , Nitta, K. , Matsumoto, Y. , Toh, K. , Miyata, K. , Uchida, S. , Nishina, K. , Osada, K. , Itaka, K. , Nishiyama, N. , Mizusawa, H. , Yamasoba, T. , Yokota, T. , & Kataoka, K. (2017). Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nature Communications, 8(1), 1001. 10.1038/s41467-017-00952-3 - DOI - PMC - PubMed
-
- Bahn, G. , Park, J.‐S. , Yun, U. J. , Lee, Y. J. , Choi, Y. , Park, J. S. , Baek, S. H. , Choi, B. Y. , Cho, Y. S. , Kim, H. K. , Han, J. , Sul, J. H. , Baik, S.‐H. , Lim, J. , Wakabayashi, N. , Bae, S. H. , Han, J.‐W. , Arumugam, T. V. , Mattson, M. P. , & Jo, D.‐G. (2019). NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer's models. Proceedings of the National Academy of Sciences, 116(25), 12516–12523. 10.1073/pnas.1819541116 - DOI - PMC - PubMed
-
- Burbulla, L. F. , Song, P. , Mazzulli, J. R. , Zampese, E. , Wong, Y. C. , Jeon, S. , Santos, D. P. , Blanz, J. , Obermaier, C. D. , Strojny, C. , Savas, J. N. , Kiskinis, E. , Zhuang, X. , Krüger, R. , Surmeier, D. J. , & Krainc, D. (2017). Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson's disease. Science, 357(6357), 1255–1261. 10.1126/science.aam9080 - DOI - PMC - PubMed
-
- Burns, J. A. , Kroll, D. S. , Feldman, D. E. , Kure Liu, C. , Manza, P. , Wiers, C. E. , Volkow, N. D. , & Wang, G.‐J. (2019). Molecular imaging of opioid and dopamine systems: Insights into the pharmacogenetics of opioid use disorders. Frontiers in Psychiatry, 10, 626. 10.3389/fpsyt.2019.00626 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

