Engineering the Reaction Pathway of a Non-heme Iron Oxygenase Using Ancestral Sequence Reconstruction
- PMID: 39642058
- PMCID: PMC11957380
- DOI: 10.1021/jacs.4c08420
Engineering the Reaction Pathway of a Non-heme Iron Oxygenase Using Ancestral Sequence Reconstruction
Abstract
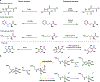
Non-heme iron (FeII), α-ketoglutarate (α-KG)-dependent oxygenases are a family of enzymes that catalyze an array of transformations that cascade forward after the formation of radical intermediates. Achieving control over the reaction pathway is highly valuable and a necessary step toward broadening the applications of these biocatalysts. Numerous approaches have been used to engineer the reaction pathway of FeII/α-KG-dependent enzymes, including site-directed mutagenesis, DNA shuffling, and site-saturation mutagenesis, among others. Herein, we showcase a novel ancestral sequence reconstruction (ASR)-guided strategy in which evolutionary information is used to pinpoint the residues critical for controlling different reaction pathways. Following this, a combinatorial site-directed mutagenesis approach was used to quickly evaluate the importance of each residue. These results were validated using a DNA shuffling strategy and through quantum mechanical/molecular mechanical (QM/MM) simulations. Using this approach, we identified a set of active site residues together with a key hydrogen bond between the substrate and an active site residue, which are crucial for dictating the dominant reaction pathway. Ultimately, we successfully converted both extant and ancestral enzymes that perform benzylic hydroxylation into variants that can catalyze an oxidative ring-expansion reaction, showcasing the potential of utilizing ASR to accelerate the reaction pathway engineering within enzyme families that share common structural and mechanistic features.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



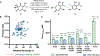

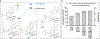
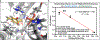
References
-
- Khersonsky O; Tawfik DS Enzyme Promiscuity: A Mechanistic and Evolutionary Perspective. Annu. Rev. Biochem. 2010, 79, 471–505. - PubMed
-
- Bornscheuer UT; Kazlauskas RJ Catalytic Promiscuity in Biocatalysis: Using Old Enzymes to Form New Bonds and Follow New Pathways. Angew. Chem., Int. Ed. 2004, 43, 6032–6040. - PubMed
-
- Baier F; Copp JN; Tokuriki N Evolution of Enzyme Superfamilies: Comprehensive Exploration of Sequence-Function Relationships. Biochemistry 2016, 55, 6375–6388. - PubMed
-
- Jensen RA Enzyme Recruitment in Evolution of New Function. Annu. Rev. Microbiol. 1976, 30, 409–425. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

