Understanding paralogous epilepsy-associated GABAA receptor variants: Clinical implications, mechanisms, and potential pitfalls
- PMID: 39642202
- PMCID: PMC11648851
- DOI: 10.1073/pnas.2413011121
Understanding paralogous epilepsy-associated GABAA receptor variants: Clinical implications, mechanisms, and potential pitfalls
Abstract
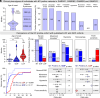
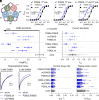
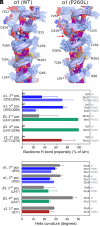
Recent discoveries have revealed that genetic variants in γ-aminobutyric acid type A (GABAA) receptor subunits can lead to both gain-of-function (GOF) and loss-of-function (LOF) receptors. GABAA receptors, however, have a pseudosymmetrical pentameric assembly, and curiously diverse functional outcomes have been reported for certain homologous variants in paralogous genes (paralogous variants). To investigate this, we assembled a cohort of 11 individuals harboring paralogous M1 proline missense variants in GABRA1, GABRB2, GABRB3, and GABRG2. Seven mutations (α1P260L, α1P260S, β2P252L, β3P253L, β3P253S, γ2P282A, and γ2P282S) in α1β2/3γ2 receptors were analyzed using electrophysiological examinations and molecular dynamics simulations. All individuals in the cohort were diagnosed with developmental and epileptic encephalopathy, with a median seizure onset age of 3.5 mo, and all exhibited global developmental delay. The clinical data for this cohort aligned with established GABAA receptor GOF but not LOF cohorts. Electrophysiological assessments revealed that all variants caused GOF by increasing GABA sensitivity by 3- to 23-fold. In some cases, this was accompanied by LOF traits such as reduced maximal current amplitude and enhanced receptor desensitization. The specific subunit mutated and whether the mutation occurred in one or two subunits within the pentamer influenced the overall effects. Molecular dynamics simulations confirmed similar structural changes from all mutations, but with position-dependent asymmetry. These findings establish that paralogous variants affecting the 100% conserved proline residue in the M1 transmembrane helix of GABAAR subunits all lead to overall GOF traits. The unexpected asymmetric and mixed effects on receptor function have broader implications for interpreting functional analyses for multimeric ion-channel proteins.
Keywords: GABRA1; GABRB3; GABRG2; epilepsy; neurodevelopmental disorders.
Conflict of interest statement
Competing interests statement:P.C. is Executive Vice President, Research at the company Saniona in Denmark. The remaining authors declare no competing interests.
Figures



References
-
- Chua H. C., Chebib M., GABA(A) receptors and the diversity in their structure and pharmacology. Adv. Pharmacol. 79, 1–34 (2017). - PubMed
-
- Maljevic S., et al. , Spectrum of GABAA receptor variants in epilepsy. Curr. Opin. Neurol. 32, 183–190 (2019). - PubMed
-
- Musto E., et al. , GABRA1-related disorders: From genetic to functional pathways. Ann. Neurol. 95, 27–41 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

